Volume 6 - Year 2018 - Pages 21-28
DOI: 10.11159/ijtan.2018.004
The iNAPO Project: Biomimetic Nanopores for a New Generation of Lab-on-Chip Micro Sensors
Wolfgang Ensinger1, Mubarak Ali1,2, Saima Nasir1, Ivana Duznovic1, Christina Trautmann1,2, Maria Eugenia Toimil-Molares2, Giuseppa R. Distefano1,2, Bodo Laube1, Max Bernhard1, Melanie MikoschWersching1, Helmut F. Schlaak1, Mario El Khoury1
1Technische Universität Darmstadt Karolinenplatz 5, 64289 Darmstadt, Germany ensinger@ma.tu-darmstadt.de
2GSI Helmholtz-Zentrum für Schwerionenforschung
Planckstrasse 1, 64291 Darmstadt, Germany
Abstract - In nature, ion conducting nanopores play a vital role for the function of living cells. They undergo gating processes where they open and close upon an external stimulus, such as the presence of a particular biomolecule, the ligand. When the gating process is observed and is quantitatively measured, one can derive data about the presence and the amount of the ligand. Hence, the nanopores can be utilized for specific sensing. However, biological nanopores are embedded in a biological cell membrane that is fragile and unstable with respect to storage and application. The iNAPO (ion conducting nanopores) project aims at combining robust polymer-based nanopores with protein-based biological nanopores, thus combining the selectivity and sensitivity of the latter with the stability and processibility of the first ones. This paper describes the different steps in the fabrication of ion conducting nanopores. It begins with ion irradiation of polymer foils, combined with chemical etching of the ion damage tracks into nanopores. By means of chemical coupling reactions, the nanopore walls are functionalized with particular molecules which react or bioconjugate with the molecules to be analyzed. As an example, a recent result on sensing a physiologically active phosphorus-based anion is shown. By means of a complexation reaction with Zn-di(picolyl)amine, the selective measurement of the concentration of the anion pyrophosphate is demonstrated. In the final step of the project, the nanopores will be incorporated into a Lab-on-Chip system for applications in e.g. medical diagnostics and environmental analysis.
Keywords: Ion Track Etching, Polymer Membrane, Nanopores, Biomimetic Sensor, Nanosensor, CurrentPotential Measurements.
© Copyright 2018 Authors - This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2017-11-25
Date Accepted: 2018-03-02
Date Published: 2018-05-31
1. Introduction
Nanopores (or –channels) in biological membranes are highly efficient biomolecular constructions, based on proteins. They control mass transport between the cell interior and its surrounding, including small molecules, water and ions [1]. Despite an often very high transport rate, the nanopores are able to discriminate between different species, i.e. let the one pass and the other one not. This gating process regulates the flow across the membrane and is crucial for the cell functionality. There are several factors that can modulate the transport through the nanopores, depending on the nanopore type, such as mechanical stress, electrical voltage and interaction with specific molecules, the ligands. The latter, for instance, can interact with a specific part of the nanopore and thus open it. Based on the effects of the mentioned gating factors, the observation of the gating process itself can, in turn, be used to monitor the gating factor, such as the presence of the ligand [2]. In other words, if it is observed that the nanopore is in an open state by measuring the mass transport (in case of ion conducting nanopores, it is the ion flow), the ligand must be present. In this way, the nanopore itself acts as a specific sensor [3]. However, there is a drawback for the application of this type of biological sensor: biological protein-based nanopores/nanochannels have been developed throughout the long-lasting evolution of cells within a biological environment, i.e. within the cell membrane. This renders them fragile, being not suitable for technical environment and application, including aspects such as storage over a longer time period or the integration into electrical devices. It would therefore be desirable to fabricate tailored nanopores from robust materials, such as polymers. This has, indeed, been achieved [2,4,5]. However, the selectivity and sensitivity for being able to sense molecular species of interest at very low concentrations and in the presence of other similar molecules are still much better for the biological protein-based nanopores in comparison to the polymer-based ones. Hence, the idea of the iNAPO (ion-conducting Nanopores) project is to combine biological protein-based nanopores with polymer-based nanopores. In a further step, they will be integrated into a lab-on-chip system in a micro-device, with a low electrical power consumption and a sensitive readout system, so that eventually a new generation of a micro-tool for (bio)chemical analysis and medical diagnostics is available. The steps of the project are shown in Fig. 1.
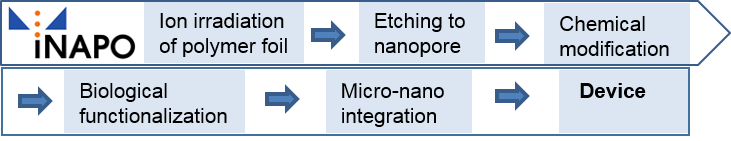
The iNAPO project is based on the expertise of physicists, material scientists, chemists, biologists and electro technicians. It is a bottom-up multi-scale approach. The final iNAPO sensing device is built up from a molecular base of the polymer foil and its modification and functionalization into a micro device in the macro world.
The target applications are in the fields of medical diagnostics and environmental analysis, e.g. for tumor marker determination in human body liquids and for measuring toxic components in ground water and drinking water.
In the following, the structure and techniques of the iNAPO project are described and a recent result is given as an example.
2. Fabrication of Polymeric Nanopores and Their Device-Integration
2. 1. Ion Irradiation: Formation of an Ion Damage Track
The first step in the fabrication of a nanopore in a polymer foil is the irradiation of the foil with a very highly energetic ion of a heavy element, such as gold. The irradiations are performed at the Universal Linear Accelerator UNILAC of the GSI Helmholtz-Zentrum für Schwerionenforschung (Center for Heavy Ion Research). Gold is vaporized into atoms in the gas phase and ionized into a positively charged state. The gold ions are accelerated in high-frequency electrostatic fields to an extreme high kinetic energy, with a velocity of about 15% of the speed of light. This extreme energy enables the ion to penetrate a polymer foil in a straight line. Mostly, a large number of ions is used. The ion fluences for multi-pore membranes typically range from 104 to 109 ions/cm2. In contrast, for sensing, a single ion, leading to a single nanopore, is utilized. As the ion beam comes along like a dense rain shower, with a large number of ions per unit square, a so-called single shot experiment is, in fact, not easy to manage. In order to create a single ion track only, a metal foil is placed in front of the polymer foil. It is thick enough to fully stop the ions. It contains an aperture of 100 µm in diameter. Next, the ion beam, which is usually focused for beam transport, is defocused so that it occupies a large diameter. This means that the number of ions per unit area becomes much smaller. Consequently, the probability that only a single ion passes the aperture is enhanced. When the single ion has passed the aperture and the polymer foil, it creates secondary electrons. When these are detected, the ion beam is immediately deflected so that it can no longer hit the foil. Thus, a single ion track can be obtained.
For the project, polyester foils are used, namely polycarbonate (PC) and polyethylene terephthalate (PET). These are materials with a wide range of applications, such as optical data storage discs (CDs, DVDs) or the well-known PET bottles. They have several advantages for the iNAPO project: they are well disposable, reasonably prized, and they have an appropriate chemistry both for the nanopore fabrication and for the nanopore modification and functionalization. The foils are being used in a thickness range between 12 and 30 µm which is well manageable and processible.
Along its trajectory, the ion projectile induces electronic excitation and ionization processes, which finally result in extended electron cascades. As a result of these processes, covalent bonds between the atoms of the polymeric network are cut, leading to severe radiation damage. Small volatile degradation products including hydrogen, hydrocarbons and carbon oxides, migrate out of the foil into vacuum, leaving back a cylindrical so-called latent ion track, a zone with reduced density and modified chemical reactivity [7,8].
2. 2. Nanopore Formation by Ion-Track Etching
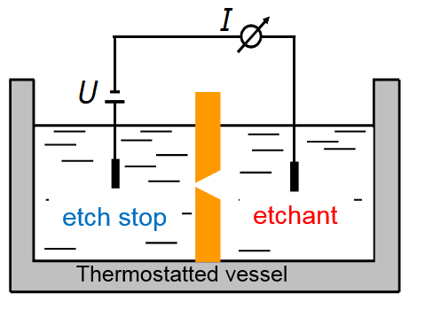
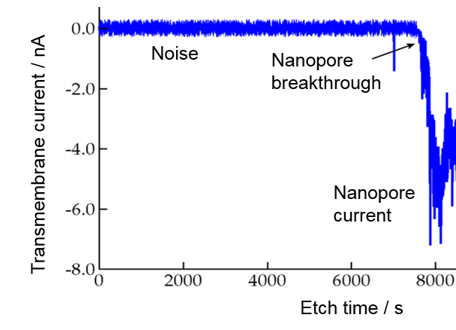
When the irradiated polymer foil is immersed into a solution of an alkaline etchant, the material around the ion track is preferentially etched away, with a rate much larger than the bulk etch rate of undamaged polymer. Thus, the ion track is developed into a cylindrical or a biconical nanopore. For sensing application, however, a conical nanopore is more favourable. Fig. 2 shows schematically the set-up for monitoring the etching process of a single conical nanopore [6,9]. In the two-compartment etch cell, the polymer foil with a single ion track is placed in the cell center, separating the two volumes. One side contains the etchant, a solution of e.g. 9 M NaOH, the other one contains an acidic etch stop, e.g. 1M formic acid in 1 M KCl solution. The ion track is dissolved from one side, finally converting the track into an open pore. The monitoring of the etching process is done by applying a potential, usually 1 V, between two gold electrodes inserted in each compartment. Since the polymer is an electrical insulator, no current flows, until the moment of pore breakthrough, when a sudden increase of the current indicates that the nanopore formation process is finished (Fig. 2). The diameter d of the small opening of the nanopore, often in the sub10 nm range, is given by Ohm’s law, based upon the known conductivity κ of the etchant solution and the nanopore dimensions:
R = 4 L / p k D d
R: resistance; L: length of nanopore = thickness of polymer foil; k: conductivity of KCl solution; D: diameter of large nanopore aperture; d: diameter of small nanopore aperture
The small opening d is defined by the etching time beyond the breakthrough point. Typically, d is around 10 nm or below, while the large aperture D being several 100 nm wide.
2. 3. Functionalization of Nanopores
The functionalization of single nanopores is done in the following two ways, (i) by attaching specific molecules to the inner pore wall, leading to the so-called first generation nanopores and (ii) by attaching binding proteins as a base for biological protein-based nanopores, representing the second-generation nanopores for sensing devices. So far, the first generation functionalization has already been achieved and an example will be shown, while the second generation is in progress with a schematic depicted below for illustration.
Upon chemical etching, the polymer backbone of a polyester is cleaved at the ester groups, leaving back a terminal carboxyl group at the nanopore surface [10]. This is shown in Fig. 3.
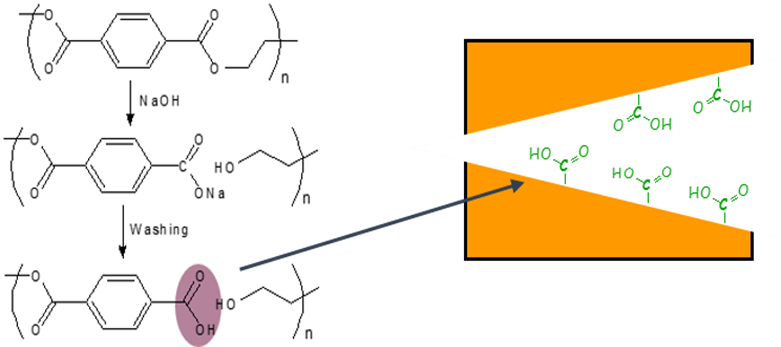
Carboxyl groups show chemical reactivity to a variety of reactions. Among them is the EDC/PFP coupling reaction that allows for bonding molecules with an amino group. The product is a modified nanopore surface, based on a stable amide bond. The preactivation of the carboxyl group is achieved with dimethylaminopropyl-ethylcarbodiimide (EDC) which reacts further to the penta fluoro phenyl (PFP) ester for a final coupling with an amine. The reaction is shown in detail in Fig. 4. Eventually, the nanopore surface carries a molecular group R. This molecule can be the counterpart to another molecule, the one to be analysed. This analyte molecule specifically reacts with R, the receptor.
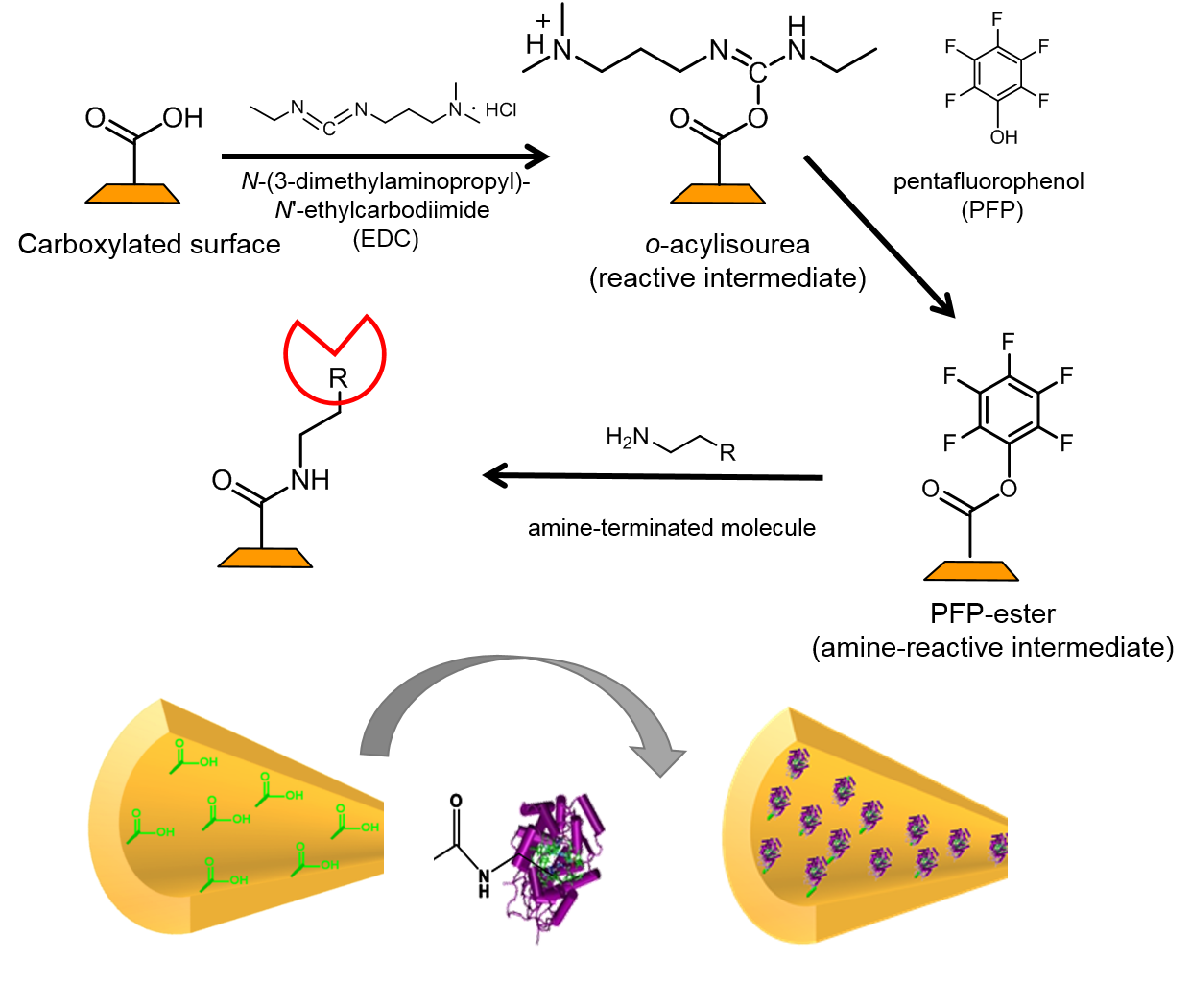
By means of the EDC/PFP amido reaction, various molecules have been coupled to the nanopore surface, including mannose, a small carbohydrate, or horseradish peroxidase, an enzyme [11,12]. Apart from coupling via covalent bonding, also non-covalent functionalization techniques have been used, such as self-assembly reactions with amphiphilic polymers [13].
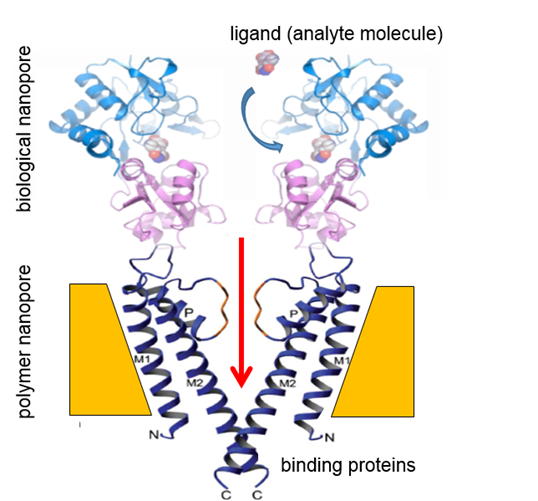
The next step in the project will be to insert binding proteins into the nanopores in order to realize biological nanopores. This is schematically shown in Fig. 5.
2. 4. Sensing Principle
Fig. 5 shows already the essential principle of a sensing device. The biomolecule (ligand) to be analysed reacts in a specific bioconjugation reaction with the receptor. In case of the biological nanopores, this reaction leads to a change in the nanopore shape, yielding an open nanopore configuration. When the nanopore is open, the ions of the electrolyte can pass and the corresponding electrical current can be measured. This gating is a stochastic event. The state of being open or closed is measured as a function of time. However, this second generation sensing combination of polymeric and biological nanopores is yet to be realized.
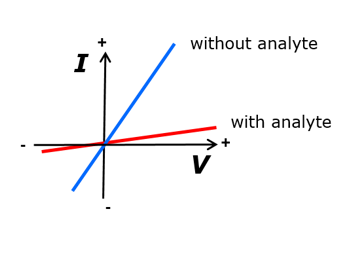
An example for the first generation sensor set-up is discussed in the following. Its functionality is schematically depicted in Fig. 6. As shown above, the wall of the nanopore is functionalized with a biorecognition molecule, the receptor R. The measurement setup is the same as shown in Fig. 2. The polymer foil with the nanopore is placed in the center of a two-compartment electrochemical cell. The cell is filled with an aqueous salt solution, such as 0.1 M KCl. This solution exhibits electrical conductivity. When a voltage is applied between the two electrodes, an ionic current flows through the nanopore. When the nanopore surface is electrically neutral, the flow is the same in both directions, irrespective of the polarity of the electrodes. If, however, the surface is charged, the asymmetric shape of the conical nanopore with its potential distribution allows for a larger ion flow in one direction in comparison to the other one. The nanopore becomes a nanofluidic diode, rectifying the current to a certain extent. The resulting current/voltage curve shows a steep line for a positive applied voltage and a flat one under negative voltage, or vice versa, depending on the sign of charge of the nanopore surface.
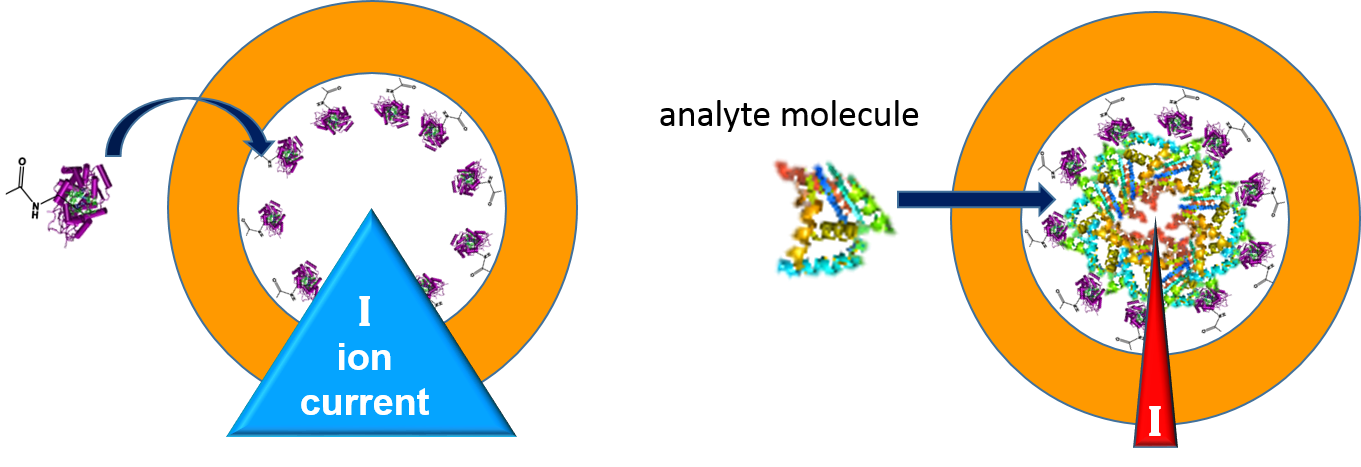
In biological nanopores, such reactions will be even more specific and sensitive. They will make use of findings from nature. As an example, glutamate recognition by a pentameric ligand-gated ion channel has recently been described [14], and it has been shown which specific domain of the N-methyl-D-aspartate receptor is responsible for activating it by the presence of glycine so that the ion-conducting nanopore opens and ions are able to flow [15].
2. 5. Integration into a Micro Device
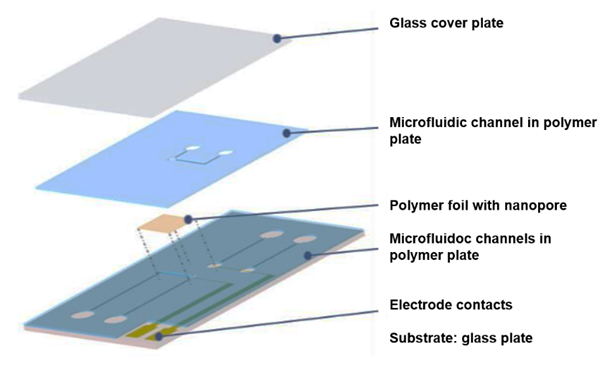
So far, laboratory set-ups have been used for the measurements. They consist of an electrochemical cell of some 10 to 15 cm length, connected via cables to a voltage source and a sensitive current meter. The project iNAPO comprises the fabrication of a compact lab-on-chip micro device with the polymer foil embedded in it by means of micro-nano-integration techniques. The polymer foil containing the nanopore is embedded into a microfluidic system with integrated electrodes, see Fig. 7. The foil is glued between two 200 µm thick sheets of the Epoxy-based photoresist SUEX containing three microfluidic channels formed by photolithography. The lower polymer plate is placed on a glass substrate with two evaporated gold electrodes; the upper plate is covered with a glass plate, as shown in Fig. 8. Details can be found elsewhere [16].
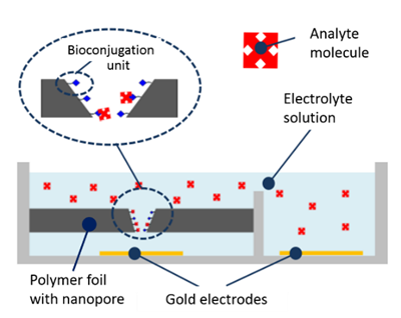
For operation, the microdevice is connected to a power supply and an amperometer, which are located in a compact unit where the microdevice is fitted in. The microchannels are filled with electrolyte, and an amperometric reference value is recorded at a given potential (typically a few 100 mV). Then, the electrolyte with analyte molecules is added and the current response is measured again. In a calibrated system, the concentration of the analyte can be determined quantitatively, if it is within the measurement range of the system.
3. Example for Molecule Sensing
The example of a nanopore sensor of the first generation deals with the analysis of pyrophosphate (P2O74- = PPi), an anion that plays an important role in biochemistry [17]. In elevated quantities, it may cause various diseases, such as disordered calcification that may lead to arthritis [18]. Therefore, monitoring is of importance. For a nanopore-based PPi-sensor, di(2-picolyl)amine moieties were coupled to the nanopore wall via the above mentioned carbodiimide-based coupling reaction. The polymer foil was exposed to an ethanolic solution of a mixture of 0.1 M EDC and 0.2 M PFP for 1 hour. Next, a reaction with a 40 mM anhydrous ethanolic solution of bis(DPA)–NH2 was carried out, followed by complexation with Zn(II) ions by means of a 0.1 mM solution of zinc chloride. The resulting bis(Zn2+-DPA) complex specifically reacts with PPi while there is no reaction with other phosphates such as monohydrogen phosphate (HPO42–), dihydrogen phosphate (H2PO4–), and adenosine mono-, di- and tri-phosphate (AMP, ADP, ATP). Fig. 9 shows schematically the complexation reaction between the zinc-DPA complex and the pyrophosphate (in green).
As mentioned above, for the sensing measurement, the foil with the single nanopore was fixed between the two halves of the conductivity cell. The electrolyte consisted of 0.1 M KCl solution with 10 mM tris-buffer in order to stabilize the solution at pH 8.0. Into each half-cell solution an Ag/AgCl electrode was inserted. A triangle voltage from −2 to +2 V was applied in small increments between the two electrodes, and the resulting trans-pore ionic current was measured. PPi and other phosphate anions such as monohydrogen phosphate, dihydrogen-phosphate, AMP, ADP, and ATP were prepared in a 0.1 M KCl solution with the before mentioned tris-buffer. The corresponding I/V curves were recorded.
The ion current through the nanopores is hardly changed when various phosphate ions are added to the electrolyte solution. In contrast, the addition of PPi causes a strong reduction of the current at negative voltages, and an increase at the positive voltage branch, see Fig. 10.
These effects are caused by the coupling of PPi with its 4 negative charges that influence the cation flow, leading to the rectification effect, as mentioned before. Hence, while the presence of other phosphates does merely change the nanopore current, PPi can be measured specifically, even down to submicromolar quantities. Further details are to be found elsewhere [19].
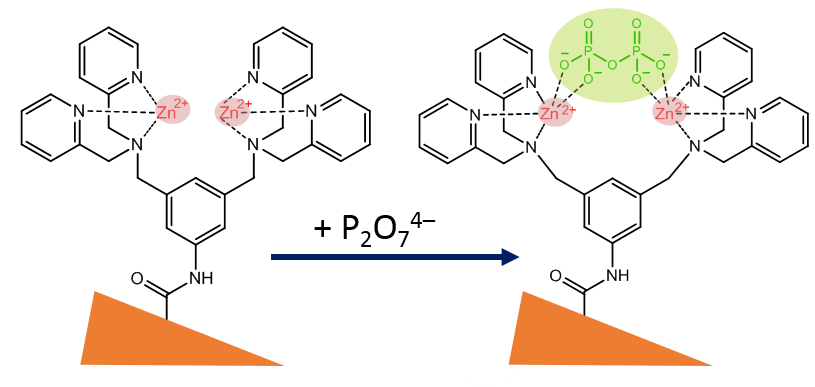
Table 1: Nanopore sensing reactions and analyte species.
| Bioconjugation/ reaction |
Bioreceptor / reactand |
Analyte | Ref. |
| Streptavidin + Biotin | Biotin | Streptavidin (protein) | [20] |
| Aptamer + Lysozyme | DNA-Aptamer (LyzAp-NH2) | Lysozyme (enzyme) | [21] |
| Mannose + Concanavalin | p-aminophenyl α-D-mannopyranoside | Concanavalin A (lectin) | [11] |
| Enzyme + H2O2 | Horseradish peroxidase HRP (Enzyme) | Hydrogen peroxide H2O2 | [12] |
| F-induced cleavage | Fcn-TBDPS-NH2 | Fluoride F- | [22] |
| Ni-Histamine complexation | Nitrilotriacetic-Ni(II) chelate | Histamine (neurotransmitter) | [23] |
| Cs+-Calixarene–crown ether complexation | p-tert-butylcalix[4]arene-crown | Cesium Cs+ | [24] |
| BSA–tryptophan | Bovine serum albumin | Tryptophan (l-Trp enantiomer) | [25] |
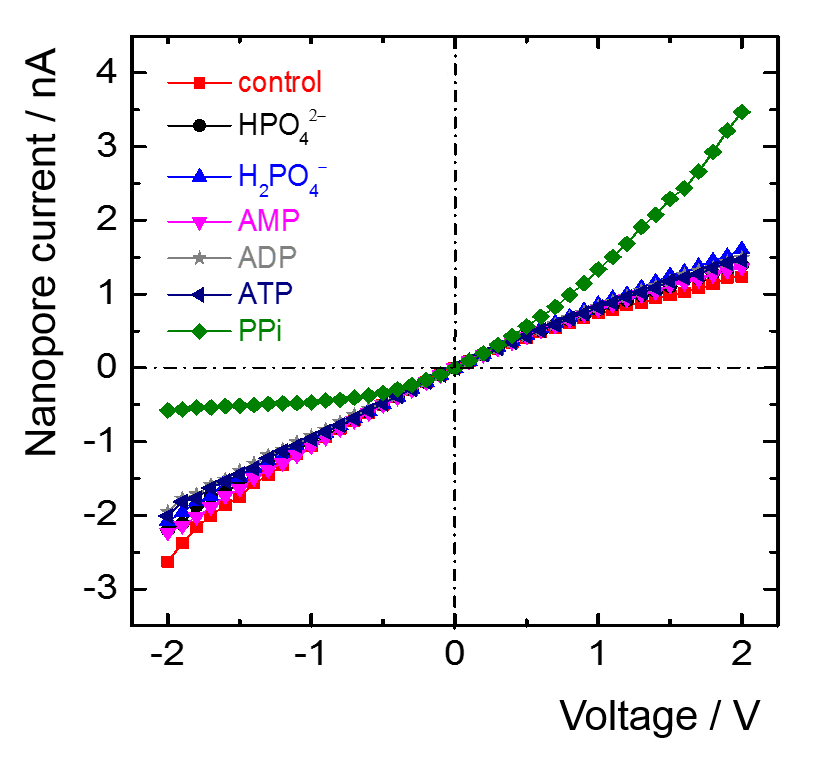
Table 1 lists further examples of nanopore-based sensing. The analyte list comprises large biomolecules such as proteins as well as small molecules and inorganic anions and cations.
4. Conclusion
The iNAPO project with fabrication of polymeric nanopores, modification and functionalization in combination with protein-based nanochannels is a biomimetic approach that spans the bridge between nature’s ion conducting pores and polymer-based nanopores and comprises their integration in a lab-on-chip device. Not shown here are further aspects, such as accompanying analysis of ionic/fluidic transport in nanopores by Nuclear Magnetic Resonance Spectroscopy and computer simulation. The overall aim is an advanced technology for future applications in medical diagnostics and environmental and process analysis.
Acknowledgment
This work has been supported in the frame of the LOEWE project iNAPO by the Hessen State Ministry of Higher Education, Research and the Arts.
References
[1] B. Hille, Ionic channels of excitable membranes. Sinauer Associates Inc., Sunderland, MA, 2001.
[2] S. M. Iqbal, R. Bashir (Eds.), Nanopores: Sensing and Fundamental Biological Interactions. Springer, Heidelberg, New York, 2011.
[3] H. Bayley, P. S. Cremer, “Stochastic sensors inspired by biology,” Nature, vol. 413, pp. 226, 2001. [4] C. Dekker, “Solid-state nanopores,” Nature Nanotechnology, vol. 2, pp. 209, 2007. View Article
[4] C. Dekker, “Solid-state nanopores,” Nature Nanotechnology, vol. 2, pp. 209, 2007. View Article
[5] M. Taglizucchi, I. Szleifer, Chemically Modified Nanopores and Nanochannels. Elsevier Science & Technology, Amsterdam, 2016.
[6] P. Y. Apel, Y. E. Korchev, Z. Siwy, R. Spohr, M. Yoshida, “Diode-like single-ion track membrane prepared by electro-stopping,” Nucl. Instrum. Methods Phys. Res. B, vol. 184, pp. 337, 2001. View Article
[7] D. Schauries, M. D. Rodriguez, B. Afra, T. Bierschenk, C. Trautmann, S. Mudie, P. Kluth, “Size characterization of ion tracks in PET and PTFE using SAXS,” Nucl. Instr. Meth. Phys. Res. B, vol. 365, pp. 573, 2015. View Article
[8] U. H. Hossain, T. Seidl, W. Ensinger, “Combined insitu infrared and mass spectrometric analysis of high-energy heavy ion induced degradation of polyvinyl polymers,” Polymer Chemistry, vol. 5, pp. 1001, 2014. View Article
[9] Z. Siwy, P. Apel, D. Dobrev, R. Neumann, R. Spohr, C. Trautmann, K. Voss, “Ion transport through asymmetric nanopores prepared by ion track etching,” Nucl. Instr. Meth. Phys. Res. B, vol. 208, pp. 143, 2003. View Article
[10] V. B. Gupta and Z. Bashir, Chapter 7, p. 320 in: Stoyko Fakirov (ed.), Handbook of Thermoplastic Polyesters. Wiley-VCH, Weinheim, 2002.
[11] M. Ali, S. Nasir, P. Ramirez, J. Cervera, S. Mafe, W. Ensinger, “Carbohydrate-mediated biomolecular recognition and gating synthetic nanochannels,” J. Phys. Chem., vol. C117, pp. 18234, 2013.
[12] M. Ali, M. N. Tahir, Z. Siwy, R. Neumann, W. Tremel, W. Ensinger, “Hydrogen peroxide sensing with horseradish peroxidase-modified polymer single conical nanochannels,” Anal. Chem., vol. 83, pp. 1673, 2011. View Article
[13] G. Perez-Mitta, L. Burr, J. S. Tuninetti, C. Trautmann, M. E. Toimil-Molares, O. Azzaroni, “Noncovalent functionalization of solid-state nanopores via self-assembly of amphipols,” Nanoscale, vol. 8, pp. 1470, 2016. View Article
[14] T. Lynagh, R. N. Beech, M. J. Lalande, K. Keller, B. A. Cromer, A. J. Wolstenholme, B. Laube, “Molecular basis for convergent evolution of glutamate recognition by pentameric ligand-gated ion channels,” Scientific Reports, vol. 24, no. 5, pp. 8558, 2015. View Article
[15] I. Mesic, C. Madry, K. Geider, M. Bernhard, H. Betz, B. Laube, “The N-terminal domain of the GluN3A subunit determines the efficacy of glycineactivated NMDA receptors,” Neuropharmacology, vol. 105, pp. 133, 2016. View Article
[16] M. El Khoury, S. Quednau, I. Duznovic, W. Ensinger, H. F. Schlaak, “Integration of Nanochannels for Lab-on-Chip-Systems“ pp. 175, in GMM-Fachbericht 86: Mikro-Nano-Integration. VDE/VDI-Gesellschaft Mikroelektronik Mikrosystem- und Feinwerktechnik (Hrsg), Duisburg, Germany, 2016.
[17] J. K. Heinonen, Biological Role of Inorganic Pyrophosphate. Kluwer Academic Publishers, London, U.K., 2001.
[18] A. E. Timms, Y. Zhang, R. G. G. Russell, M. A. Brown, “Genetic studies of disorders of calcium crystal deposition,” Rheumatology, vol. 41, pp. 725, 2002. View Article
[19] M. Ali, I. Ahmed, P. Ramirez, S. Nasir, C. M. Niemeyer, S. Mafe, W. Ensinger, “Label-Free Pyrophosphate Recognition with Functionalized Asymmetric Nanopores,” Small, vol. 12, pp. 2014, 2016. View Article
[20] M. Ali, B. Yameen, R. Neumann, W. Ensinger, W. Knoll, O. Azzaroni, “Biosensing and Supramolecular Bioconjugation in Single Conical Polymer Nanochannels. Facile Incorporation of Biorecognition Elements into Nanoconfined Geometries,” Journal of the American Chemical Society, vol. 130, pp. 16351, 2008. View Article
[21] M. Ali, S. Nasir, W. Ensinger, “Bioconjugationinduced ionic current rectification in aptamermodified single cylindrical nanopores,” Chemical Communications, vol. 51, pp. 3454, 2015. View Article
[22] M. Ali, I. Ahmed, P. Ramirez, S. Nasir, J. Cervera, C. M. Niemeyer, W. Ensinger, “Fluoride-induced modulation of ionic transport in asymmetric nanopores functionalized with "caged" fluorescein moieties,” Nanoscale, vol. 8, pp. 8583, 2016. View Article
[23] M. Ali, P. Ramirez, I. Duznovic, S. Nasir, S. Mafe, W. Ensinger, “Label-free histamine detection with nanofluidic diodes through metal ion displacement mechanism,” Colloids and Surfaces B: Biointerfaces, vol. 150, pp. 201, 2017. View Article
[24] M. Ali, I. Ahmed, P. Ramirez, S. Nasir, J. Cervera, S. Mafe, C. M. Niemeyer, W. Ensinger, “Cesium– induced ionic conduction through a single nanofluidic pore modified with calixcrown moieties,” Langmuir, vol. 33, pp. 9170, 2017. View Article
[25] M. Ali, S. Nasir, W. Ensinger, “Stereoselective detection of amino acids with protein-modified single asymmetric nanopores,” Electrochimica Acta vol. 215, pp. 231, 2016. View Article

