Volume 3 - Year 2015 - Pages 20-27
DOI: 10.11159/ijtan.2015.003
Colloidosomes from Peroxidized Pickering Emulsions
Andriy Popadyuk1, Nadiya Popadyuk1, Ihor Tarnavchyk1, Stanislav Voronov2, Andriy Voronov1
1North Dakota State University, Coatings and Polymeric Materials,
Dep.2760, PO Box 6050, Fargo, 58105, USA
andriy.voronov@ndsu.edu
2Lviv Polytechnic National University, Department of Organic Chemistry,
Bandera St.12, Lviv, Ukraine, 79013
stanislav.voronov@gmail.com
Abstract - A new approach to synthesis of cross-linked colloidosomes (microcapsules with a shell from colloidal particles) was developed on the basis of a peroxidized Pickering emulsion (an emulsion stabilized exclusively by peroxidized colloidal particles). Peroxidized latex particles were employed to ensure formation of Pickering emulsion. Free radical polymerization was used to convert droplets of a peroxidized Pickering emulsion into colloidosomes (soft template technique).
The peroxidized latex particles were synthesized with the use of amphiphilic polyperoxide copolymer poly[N-(t-butyl-peroxymethyl) acrylamide]-co-maleic anhydride (PM-MA)applied as both initiator and surfactant (inisurf) in the emulsion polymerization process. The polymerization results in latexes with a controllable amount of peroxide and carboxyl groups (both derived from PM-MA macromolecules) at the particle surface.
It was demonstrated that the structure of the synthesized (using peroxidized latex particles) colloidosomes depends on the amount of functional groups and pH during the synthesis. Thus, the size and morphology of colloidosomes can be controlled by latex particle surface properties.
Keywords: polyperoxide copolymer, inisurf, peroxidized colloidosomes.
© Copyright 2015 Authors - This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2015-07-31
Date Accepted: 2015-11-16
Date Published: 2015-12-14
1. Introduction
It is difficult to underestimate the importance of colloidal systems in current polymer technology and the development of polymer materials including nanomaterials. To this end, constant efforts are being made in widely used in industry emulsion and suspension polymerization techniques in order to improve existing processes and develop new advanced polymers and polymer materials. Such polymerization processes usually use templates from liquid droplets stabilized by molecular surfactants to yield tailored solid polymeric nanomaterials (nanoparticles).
Over the past decade, considerable attention has been focused on surfactant-free systems [1], as they provide an opportunity to reduce the environmental impact of polymer materials. More specifically, great attention has been drawn to Pickering emulsions, stable colloidal systems of two immiscible liquids, one of which is dispersed in the other with the help of solid particles [2]-[4]. The phenomena of particle surface activity were first described by Pickering and Ramsden [5], [6]. Several decades later, Velev [7], [8] proposed a mechanism of self-assembly of colloidal particles at the liquid-liquid interphase. It was established that Pickering emulsions exhibit superior kinetic and thermodynamic stability compared to conventional emulsions. The stabilization mechanism of colloidal particles is based on the reduction of interfacial tension, similar to molecular surfactants. However, compared to surfactant molecules, colloidal particles are significantly larger and that imparts specifics to emulsification process and stability profile [9], [10]. Using a Pickering emulsion opens up a broad range of opportunities for not only improving the properties and performance of already existing polymer colloids, but also developing new polymer composite materials and nanomaterials [11].
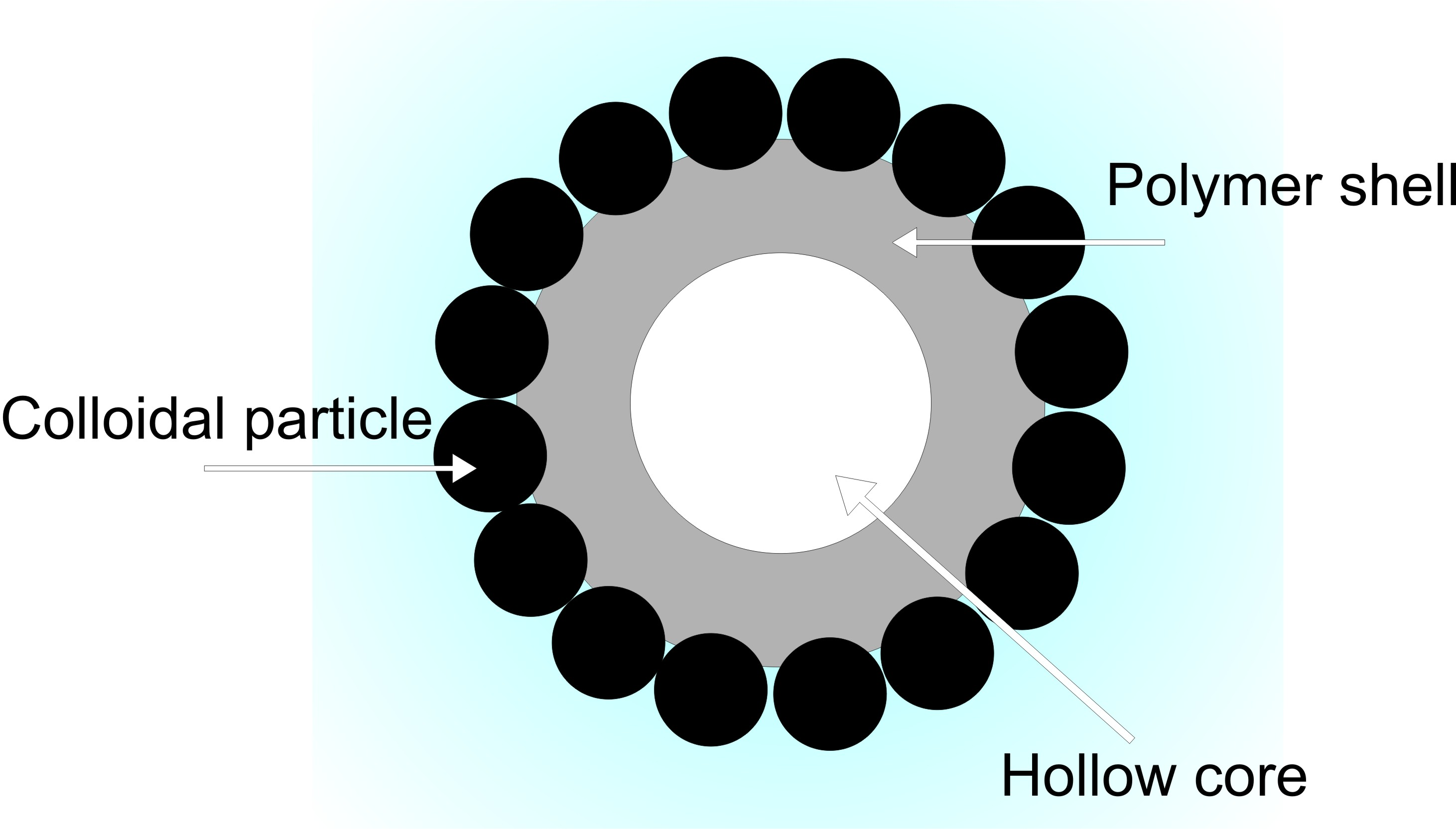
Colloidal structures obtained as a result of fully or partially solidifying droplets of Pickering emulsion are generally referred to as colloidosomes (Figure 1). To date, there are reports in the literature of the synthesis of various types of colloidosomes, including "hairy" colloidosomes [12], "sensitive" colloidosomes [13], [14], and "carbon nanotubosomes" [15]. The formation of covalently cross-linked colloidosomes via free radical and condensation polymerization mechanisms has been reported [16]-[18] as an approach for fabrication of various colloidosome morphologies, such as core-shell and capsule-like morphologies [17], [19], [20]. Because of the ability to finely tune colloidosome properties (density of particle packing at the surface, shell thickness and porosity, core type, composition, etc.), such structures can be engineered to efficiently encapsulate (absorb) and deliver (release) various cargos in a controlled fashion [21]. Reports show that colloidosomes can be employed as an effective encapsulation tool for drug delivery or protection of components in the food and cosmetics industries [22][-25].
In this study, surface-functionalized (peroxidized) latex particles were applied in the synthesis of cross-linked colloidosomes. The latexes were synthesized using an amphiphilic polyperoxide copolymer acting as an inisurf (initiator and surfactant at the same time) in the emulsion polymerization process. The employed in colloidosomes synthesis amphiphilic polyperoxide copolymer poly[N-(t-butyl-peroxymethyl) acrylamide]-co-maleic anhydride (PM-MA) was recently developed in our group [27].
It was expected that the presence of carboxyl and peroxide functional groups (both derived from inisurf molecules) on the latex particles surface would provide: i) the required surface properties of particles (hydrophilic-lipophilic balance, HLB), sustaining a Pickering emulsion mechanism for synthesis of colloidosomes [7], [20]; ii) the initiation of free radical reactions, polymer formation, grafting, and cross-linking within Pickering emulsion droplets [20]; iii) an opportunity to further functionalize colloidosomes in post-polymerization reactions [20], [27].
It was demonstrated that the presence of functional groups on latex particle surfaces plays a crucial role in the stability of a peroxidized Pickering emulsion (by controlling the HLB) and determines success in colloidosome synthesis. By utilizing peroxide functional groups of latex particles, colloidosomes can be further modified to obtain a covalently cross-linked shell and hollow (liquid) core.
2. Materials
Styrene (St), divinylbenzene (DVB) (Sigma-Aldrich, St. Louis, MO), hexadecane (HD) (Alfa Aesar, Ward Hill, MA), 2,2'-azobis-isobutyronitrile (AIBN) (Aldrich, St. Louis, MO). Amphiphilic copolymer poly[N-(t-butyl-peroxymethyl) acrylamide]-co-maleic anhydride (inisurf, PM-MA) was synthesized as described elsewhere [27]. All monomers were purified by vacuum distillation, other reagents and water (MiliQ, 18 MΩ) were used as received.
3. Methods
3. 1. Synthesis of Peroxidized Latexes
Emulsion polymerization was carried out in order to synthesize peroxidized latex particles. Briefly, a 10 wt. % emulsion of styrene in an aqueous solution of PM-MA (1 wt. % per styrene) was prepared at pH 7.5 by magnetic stirring at 1200 rpm for 30 min. The emulsion was purged with argon under constant stirring for 20 min and subsequently placed in an oil bath heated to 85°C. The polymerization was conducted for 4 h under constant stirring (conversion up to 95%). At the end of the reaction, the latexes were cooled and placed into a 5 mL dialysis bag (50 kDa molecular cut-off). The bag was submerged into a 2000 mL beaker filled with distilled water adjusted to pH 9.5 using NaOH. At intervals of 24 h, the water in the beaker was replaced to ensure a constant high concentration gradient to improve the dialysis process. After 14 days, the peroxidized latexes were removed from the dialysis bag and stored at 4 °C.
The resulting polymer was then either i) stored at 4 °C or ii) precipitated by a freeze-thaw cycle and washed repeatedly with 0.1 N NaOH and hexane to remove non-reacted material. The resulting polymer was collected, dried at room temperature and stored at 4 °C to be used for further analysis.
3. 2. Dynamic Light Scattering and Zeta-Potential Measurements of Peroxidized Latexes
Particle size distribution measurements were performed in dilute aqueous dispersions of latex particles using Malvern Zetasizer Nano-ZS90 at 25 °C.
The final numbers represent an average of a minimum of 5 individual measurements.
3. 3. Evaluation of Carboxyl Groups Amount on Surface of Latex Particles
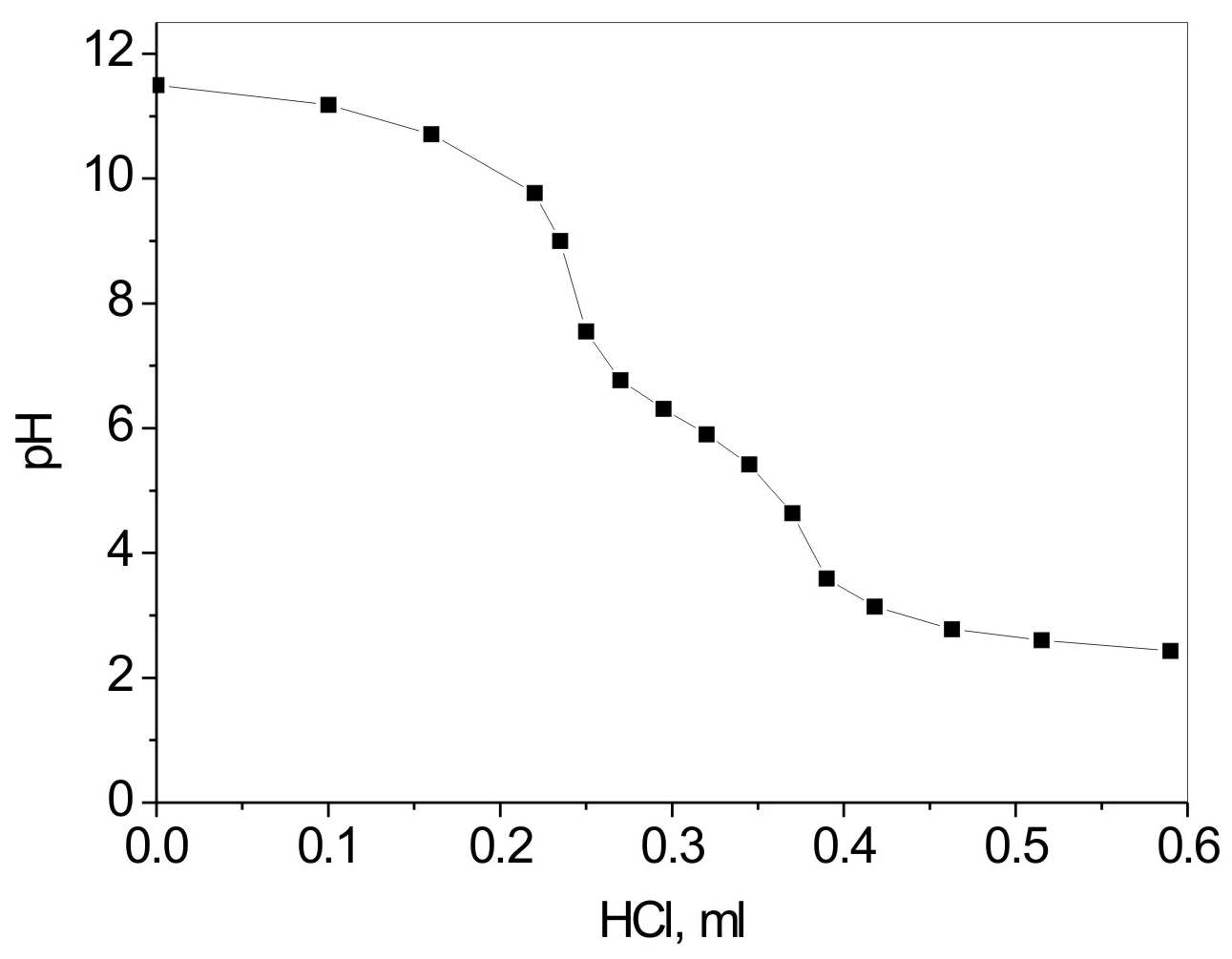
A potentiometric titration (back titration using 0.1 N HCl as a titrant) of peroxidized latexes was used to calculate the amount of carboxyl groups (originated from PM-MA) on the surface of latex particles.
3. 4. Evaluation of Peroxide Groups Amount on Surface of Latex Particles
Thermal analysis of peroxidized latexes was used to calculate the amount of peroxide groups on the surface of latex particles. For this purpose, latex samples (15 mg) were heated an underlying heating rate of 10°C/min to 400°C in air using a TA Instruments Q500.
3. 5. Colloidosomes Synthesis
Peroxidized Pickering emulsions were formed by mixing 28 g of an aqueous phase (containing 0.3 - 1 wt.% of peroxidized latex particles) with 2 g of an oil phase (consisting of HD, St, DVB, and 0.06 g AIBN initiator). Mixtures of oil and aqueous phases were homogenized at 10,000 rpm in a pulsating regime (1 pulse consisting of 60 sec of homogenizing + 20 sec of rest time) for 8 min, using a T25 Ultra-Turrax homogenizer (IKA, USA).
Obtained peroxidized Pickering emulsions were transferred into a round bottom flask, purged with argon for 20 min, and subsequently polymerized at 80°C for 24h. After polymerization, colloidosomes were isolated from reaction mixture, thoroughly washed with an acetone/ethanol (1:1) mixture, and stored either under H2O or HD layer, or in dry state.
3. 6. Colloidosomes Imaging
Digital images of latex particles were obtained using JEOL JSM-6490LV scanning electron microscopy.
4. Results and Discussion
The main goal of this work was to develop a fabrication route for colloidosome synthesis on the basis of a Pickering emulsion stabilized using peroxidized latex particles.
To achieve this goal, two objectives were detailed: i) to synthesize colloidosomes using peroxidized latex particles and ii) to investigate how the presence of hydrophilic functional groups on latex particle surfaces impacts peroxidized Pickering emulsion stability.
4. 1. Synthesis and Characterization of Peroxidized Latexes
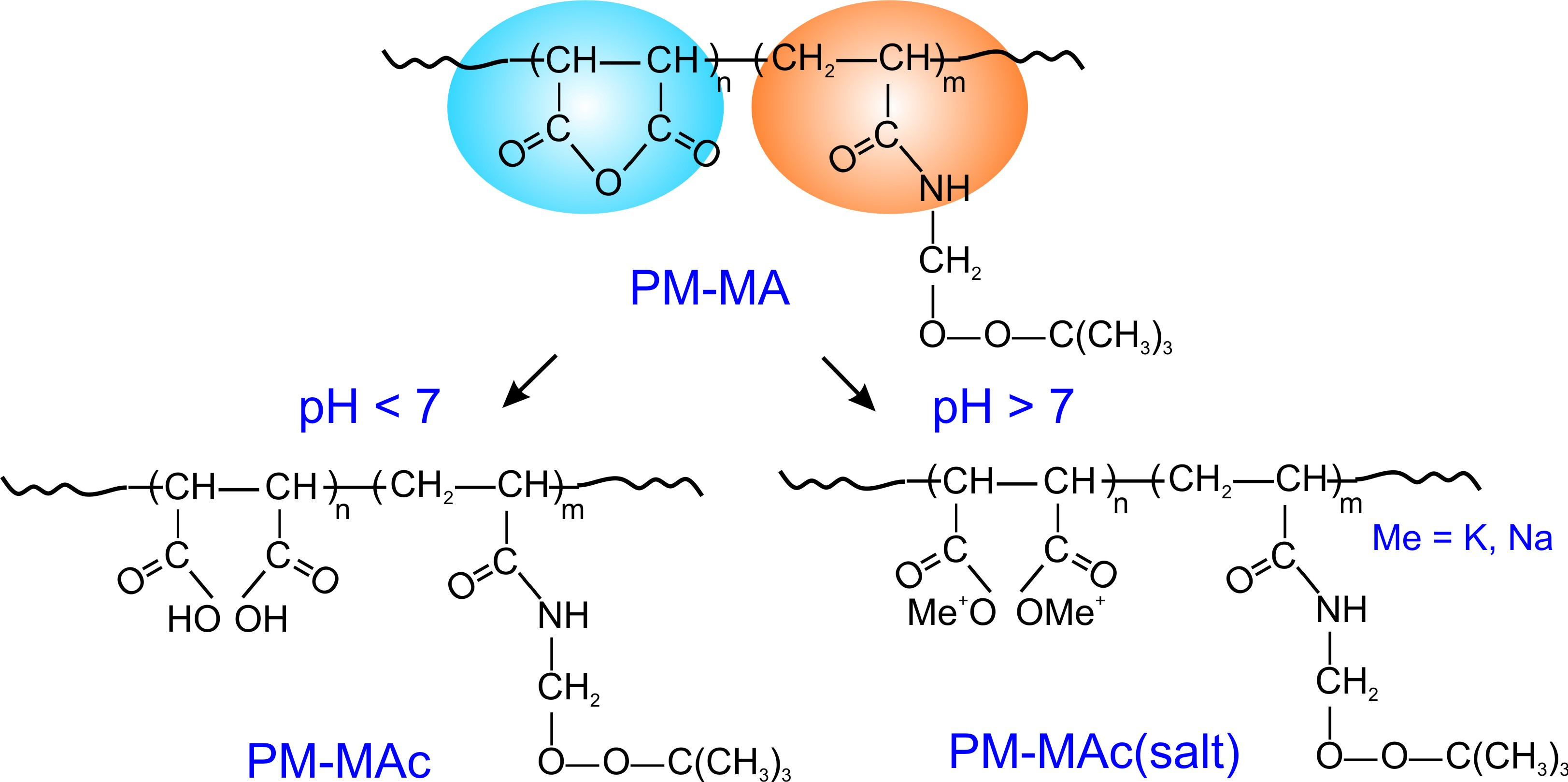
Amphiphilic PM-MA copolymer (Figure 2) was employed in emulsion polymerization to synthesize peroxidized latex particles [27]. In order to provide particles surface activity and their ability to stabilize a Pickering emulsion in colloidosomes synthesis, these particles need to exhibit amphiphilic behaviour - i.e., to combine hydrophilic and hydrophobic molecular moieties at the surface. In the case of peroxidized latexes, hydrophilic properties are provided by hydrolysis of PM-MA maleic anhydride groups (blue area, Figure 2) and formed carboxyl groups of PM-MAc at pH<7 or PM-MAc(salt) at pH>7 inisurf macromolecules. Whereas non-polar PM fragments of the copolymer (orange area, Figure 2) and polystyrene (core material of latex) provide hydrophobic properties to the particle's surface.
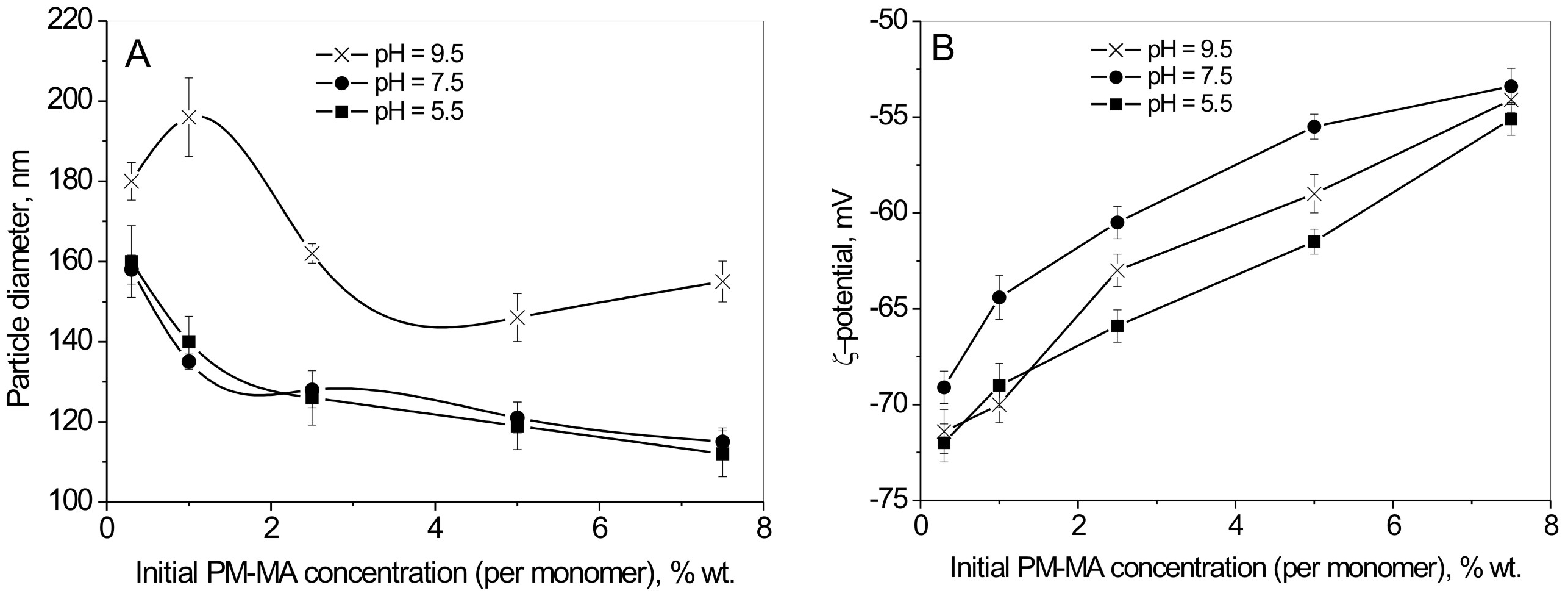
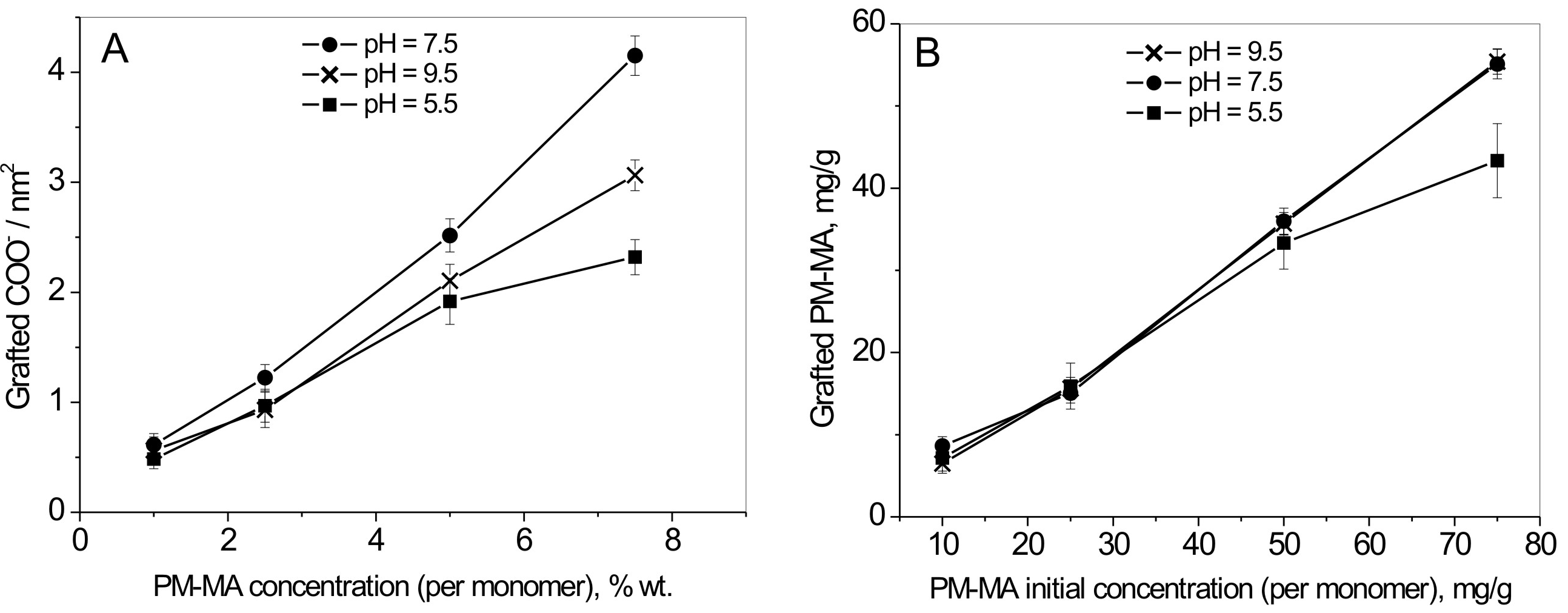
In order to investigate the ability to vary the amount of carboxyl groups on the surface of latex particles, and thus their surface activity, a series of peroxidized latexes were synthesized at pH 5.5, 7.5, and 9.5 using various PM-MA concentrations (0.3, 1, 2.5, 5 and 7.5 wt. % based on monomer weight). Figure 3A represents the size variations of the obtained at 85°C peroxidized particles as a function of pH and initial PM-MA concentration. In general, the size of latex particles decreases with an increasing PM-MA concentration and with decreasing pH. The latter can be explained by the fact that a higher concentration of inisurf obviously results in a larger number of micelles (nucleating sites for particle growth in emulsion polymerization), thus, a greater number of smaller particles is formed. HLB of inisurf macromolecules depends on the pH of the solution. At a higher pH, inisurf macromolecules are more hydrophilic, thus more of them are required to form a micelle (the opposite is true for lower pH). Consequently, the pH changes the total number of micelles in polymerization and results in variations in latex particle number and size (Figure 3A).
It was confirmed that inisurf macromolecules at elevated temperatures undergo covalent attachment (grafting) to latex particle surface during polymerization [20], [27]. Potentiometric titration of peroxidized latexes was performed in order to determine the amount of carboxyl groups on the particles' surface (Figure 4).
The obtained data reveal that only 60-80% of carboxyl groups in grafted macromolecules are indeed located on the particle surface. Thus a significant part of anhydride groups was localized in the particle core during the synthesis and may not undergo hydrolysis. Figure 1B shows the zeta potential measurements of peroxidized latexes, providing information on the overall effect of inisurf concentration and pH on the particle's charge. The data show that the amount of carboxyl groups on the surface of latex particles depends almost linearly on concentration of inisurf (Figure 5B). However, the density of the functional groups on the surface differs with inisurf concentration, possibly due to changes in latex particle size (Figure 5A).
We believe that the observed changes of zeta potential with respect to pH during synthesis can be explained by the fact that part of anhydride groups is not accessible for hydrolysis, as it was determined by potentiometric titration.
To this end, it is observed that increasing inisurf concentration in latex synthesis results in a significantly smaller size of the resulting particles and a larger amount of carboxyl groups on the surface. The size of particles is determined by pH during emulsion polymerization, and is also affected by the amount of carboxyl groups on the surface of particles.
As a result, a variable amount of carboxyl groups on the surface of peroxidized latex particles allows control over particle surface properties (including HLB).
As the next step, colloidosome synthesis was attempted using peroxidized latex particles.
4. 2. Colloidosome Preparation
This study employed the "soft template" approach, where liquid droplets of Pickering emulsion serve as templates for cross-linked colloidosomes synthesis [7].
A peroxidized Pickering emulsion was composed of an oil phase from a monomer mixture, an initiator, and a hydrophobic solvent (HD) that was emulsified in an aqueous phase, where peroxidized latex particles, exclusively acting as a surface-active ingredient, were located. Once a peroxidized Pickering emulsion was formed, it was heated at 80°C to initiate free radical polymerization inside each oil droplet, yielding hollow (HD filled) colloidosomes (Figure 6).
The formation and stability of a Pickering emulsion depend on the latex particles' surface activity (surface HLB). Therefore, the ability to control the HLB of the particle surface is crucial. To confirm that peroxidized latex particles can be used in Pickering emulsion formation for covalently cross-linked colloidosome synthesis, a series of peroxidized Pickering emulsions was prepared.
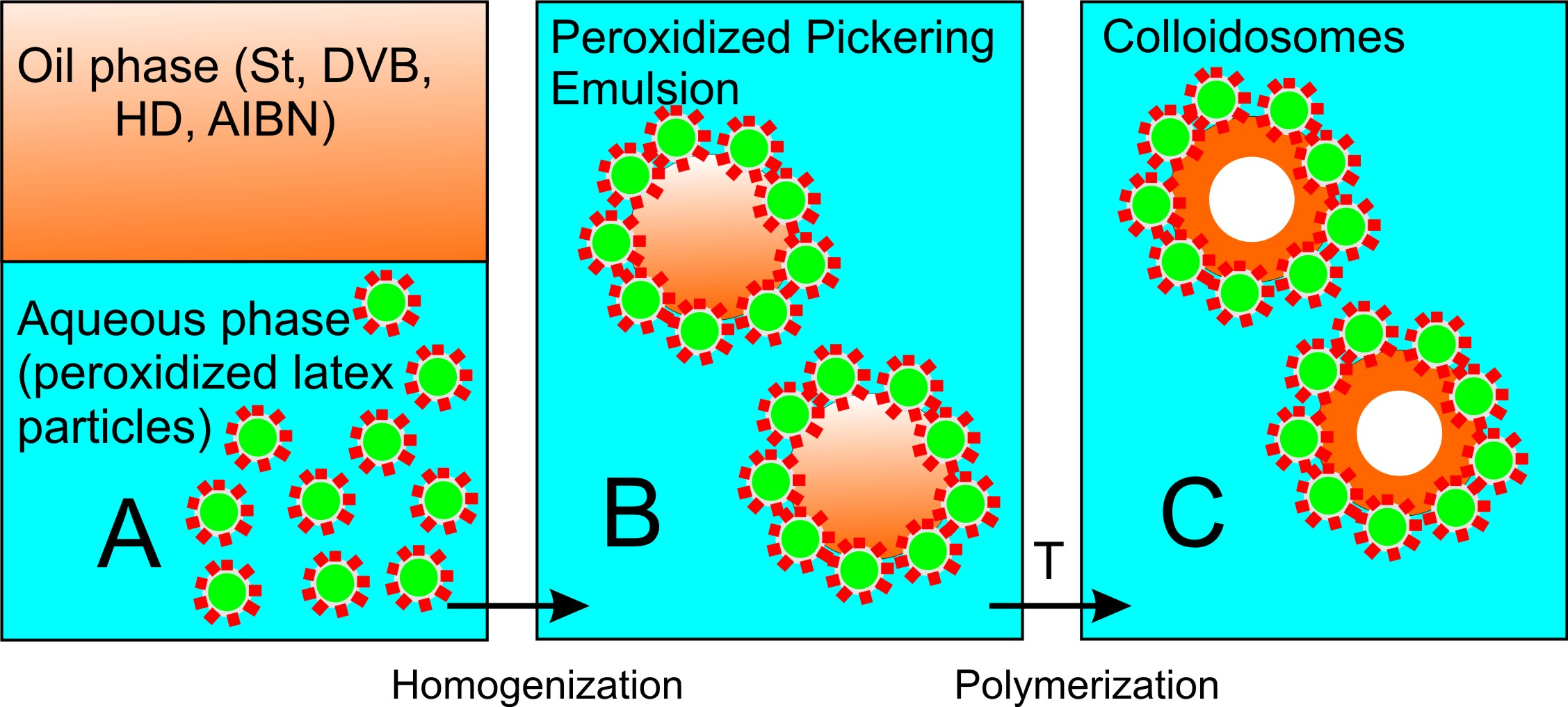
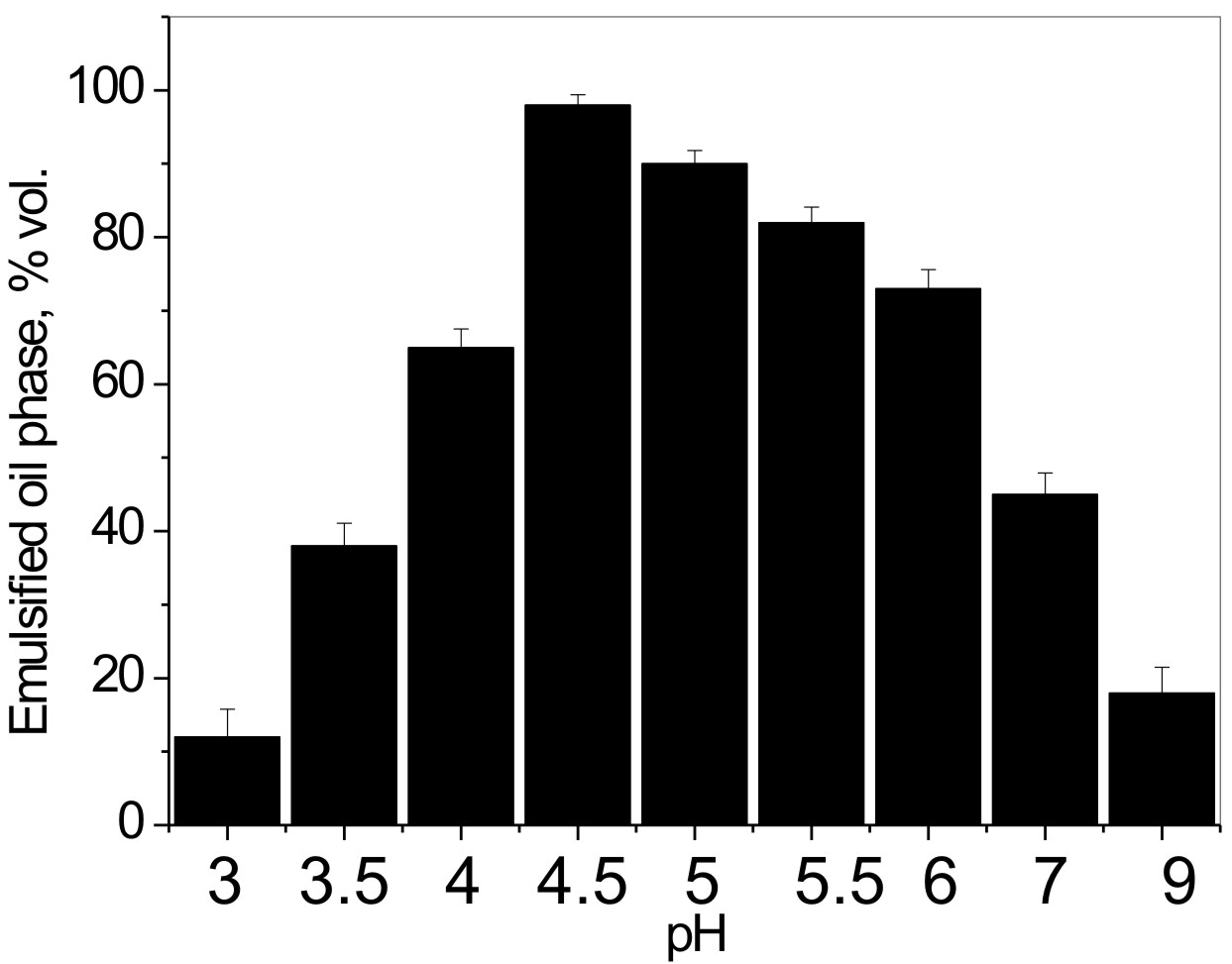
First, the formation and stability of a series of formulated peroxidized Pickering emulsions were studied as a function of pH (Figure 8). It was found that there is an optimum pH range in which superior Pickering emulsion stability can be achieved. Notably, at pH < 3 and pH > 9, no emulsion formation was observed, which can be explained by either an absence (pH < 3) or an excessive amount (pH > 9) of ionized carboxyl groups on the latex particles' surface, causing the particles to coagulate due to detrimental HLB change of inisurf macromolecules acting as particle stabilizers.
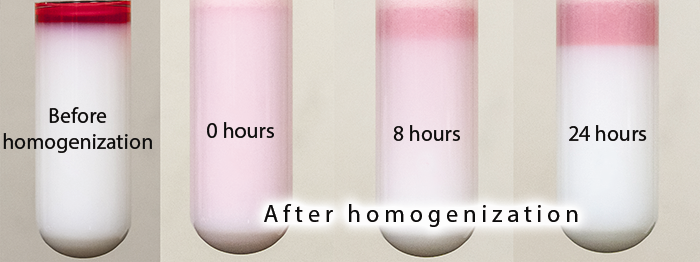
Immediately after formation, a peroxidized Pickering emulsion 'creams' by floating an emulsified oil phase on top of an excessive aqueous phase (Figure 7). This effect can be explained by the different density of the aqueous and oil phases (large size prevents droplet suspension). At this point, formulation quality can be assessed by inspecting the aqueous phase for the presence of excessive latex particles. Opalescence and turbidity would signify inefficient use of latex particles. By adjusting pH, shear rate, and concentration of latex particles, it is possible to form clear (particle-free) aqueous phase, indicating that all particles are involved in droplet stabilization.
For the synthesis of colloidosomes, latexes developed at pH 7.5 at 85°C in the presence of 1 wt. % (based on monomer weight) inisurf were chosen. It was our assumption that such latexes, while having a smaller amount of carboxyl groups, exhibit sufficient surface activity to stabilize a Pickering emulsion.
Table 1. Colloidosome synthesis conditions (at 80°C) and properties.
|
Sample |
pH |
Oil phase composition, wt. % |
Colloidosome dimensions |
||||
|
St |
DVB |
HD |
AIBN |
D, µm |
Shell, µm |
||
|
CS1 |
7.5 |
28 |
12 |
57 |
3 |
10±8 |
1.7±0.4 |
|
CS4 |
3.5 |
28 |
12 |
57 |
3 |
10±4 |
1.3±0.3 |
|
CS3 |
4.5 |
28 |
12 |
57 |
3 |
15±3 |
1.5±0.4 |
|
CS2 |
4.5 |
14 |
6 |
77 |
3 |
10±2 |
0.8±0.2 |
After formulating a peroxidized Pickering emulsion, polymerization was conducted to yield hollow colloidosomes decorated with peroxidized latex particles. Table 1 shows data on the properties of the synthesized colloidosomes. The obtained results indicate that several morphological characteristics of colloidosomes can be controlled by variations in oil phase composition (e.g., monomer/HD ratio) and the pH of the aqueous phase.
At the same time, the size of colloidosomes can be adjusted by the pH of the aqueous phase and homogenization parameters (pulse length, sonication intensity), whereas shell thickness depends on the total initial concentration of monomers.
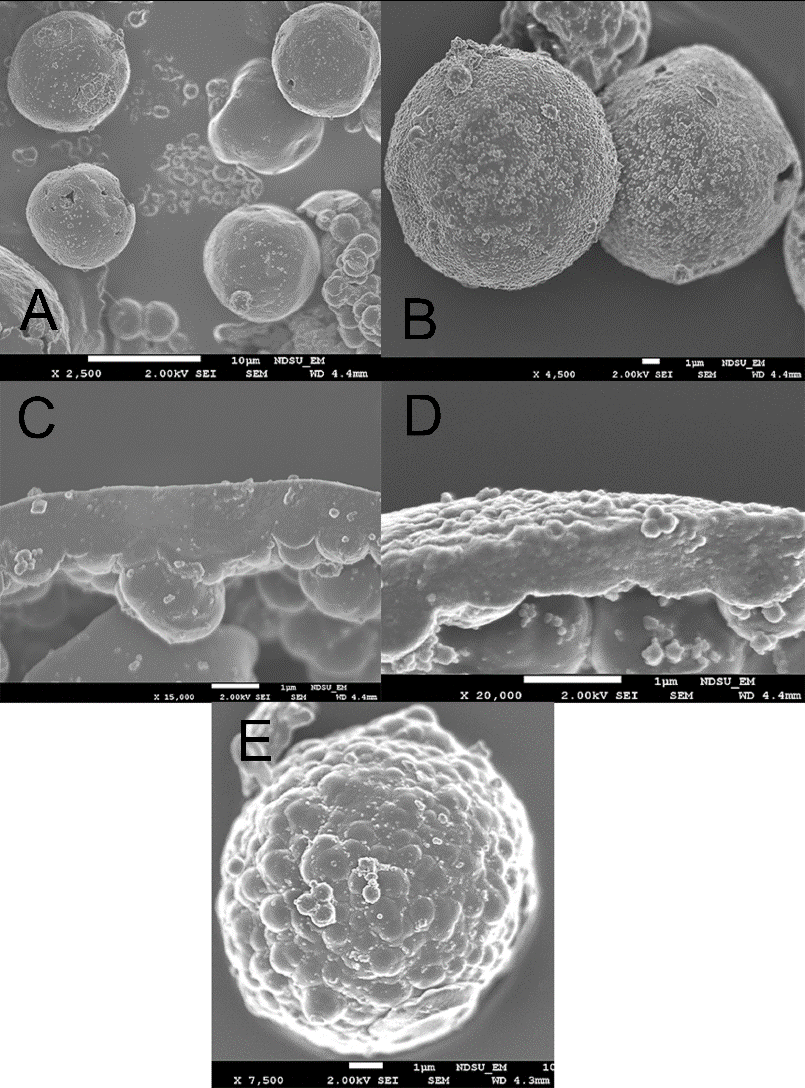
SEM measurements confirm that several different colloidosome morphologies can be synthesized using peroxidized latex particles (Figure 9).
Although the surface charge of latex particles is essential for electrostatic stabilization of droplets in a peroxidized Pickering emulsion, it can also be disruptive if it is too large. Notably, the colloidosomes prepared at pH 7.5, unlike other samples, did not feature a smooth surface covered with grafted latex particles. Their outer surface shows signs of phase separation during synthesis and an obvious lack of latex particles, possibly due to excessive particle charge at pH 7.5.
5. Conclusion
Peroxidized latex particles with variable amounts of carboxyl groups on particle surfaces were successfully employed in the formation of covalently cross-linked colloidosomes using a peroxodized Pickering emulsion polymerization approach.
It was shown that by varying the HLB of the surface of latex particles (the amount of carboxyl and peroxide groups), colloidosomes with several different morphologies can be synthesized.
It was found that the surface charge of the particles is essential for maintaining the stability of the droplets in a peroxidized Pickering emulsion and, thus, the colloidosome formation.
The described synthetic technique allows for fabrication of carrier colloidosome capsules free of surfactant contamination that can potentially leach out during colloidosome application.
References
[1] T. Sakai, "Surfactant-free emulsions," Current Opinion in Colloid & Interface Science, vol. 13, no. 4, pp. 228-235, 2008. View Article
[2] B. P. Binks, "Particles as surfactants," Current Opinion in Colloid & Interface Science, vol. 7, no. 1-2, pp. 21-41, 2002. View Article
[3] F. J. Rossier-Miranda, C. G. P. H. Schroen and R. M. Boom, "Colloidosomes: Versatile microcapsules in perspective," Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 343, no. 1-3, pp. 43-49, 2009. View Article
[4] S. Saraf, R. Rathi, C. C. D. Kaur and S. Saraf, "Colloidosomes and Advanced Vesicular System in Drug Delivery," Asia Journal of Scientific Research, vol. 4, no. 1, pp. 1-15, 2001. View Article
[5] S. U. Pickering, "Emulsions," Journal of the Chemical Society, vol. 91, pp. 2001-2021, 1907. View Article
[6] W. Ramsden, "Separation of Solids in the Surface-Layers of Solutions and 'Suspensions'," in Proceedings of the Royal Society of London, London, 1903. View Article
[7] O. D. Velev, K. Furusawa and K. Nagayama, "Assembly of latex particles by using emulsion droplets as templates. 1. Microstructured hollow spheres," Langmuir, vol. 12, no. 10, pp. 2374-2384, 1996. View Article
[8] O. D. Velev, K. Furusawa and K. Nagayama, "Assembly of latex particles by using emulsion droplets as templates. 2. Ball-like and composite aggregates," Langmuir, vol. 12, no. 10, pp. 2385-2391, 1996. View Article
[9] A. D. Dismore, M. F. Hsu, M. G. Nicolaides, M. Marquez, A. R. Bausch and D. A. Weitz, "Colloidosomes: selectively permeable capsules, composed of colloidal particles," Science, vol. 298, no. 5595, pp. 1006-1009, 2002. View Article
[10] A. B. Taubman and A. F. Koretskiy, "Stabilization of emulsions with solid surfactants and coagulative structure formation," in Achiev. of Col. Sci., Moscow, Nauka, 1976.
[11] S. H. Kim, G. R. Yi, K. H. Kim and S. M. Yang, "Photocurable Pickering emulsions for colloidal particles with structural complexity," Langmuir, vol. 24, no. 6, pp. 2365-2371, 2008. View Article
[12] P. F. Noble, O. J. Cayre, R. G. Alargova, O. D. Velev and V. N. Paunov, "Fabrication of "Hairy" Colloidosomes with Shells of Polymeric Microrods," Journal of the American Chemical Society, vol. 126, no. 26, pp. 8092-8093, 2004. View Article
[13] D. B. Lawrence, T. Cali, Z. Hu, M. Marquez and A. D. Dinsmore, "Temperature-responsive semipermeable capsules composed of colloidal microgel spheres," Langmuir, vol. 23, no. 2, pp. 395-398, 2007. View Article
[14] J. W. Kim, A. Frenandes-Nieves, N. Dan, A. S. Utada, M. Marquez and D. A. Weitz, "Colloidal assembly route for responsive colloidosomes with tunable permeability," Nano Letters, vol. 7, no. 9, pp. 2876-2880, 2007. View Article
[15] M. Panhuis and V. N. Paunov, "Assembling carbon nanotubosomes using an emulsion-inversion technique," Chemical Communications, vol. 13, pp. 1726-1728, 2005. View Article
[16] D. Yin, Q. Zhang, H. Zhang and C. Yin, "Fabrication of covalently-bonded polystyrene/SiO2 composites by Pickering emulsion polymerization," Journal of Polymer Research, vol. 17, no. 5, pp. 689-696, 2010. View Article
[17] T. Chen, P. J. Colver and S. A. F. Bon, "Organic-inorganic hybrid hollow spheres prepared from TiO2-stabilized Pickering emulsion polymerization," Advanced Materials, vol. 19, no. 17, pp. 2286-2289, 2007. View Article
[18] K. L. Thompson, S. P. Arnes, J. R. Howse, S. Ebbens, I. Ahmad, J. H. Zaidi, D. W. York and J. A. Burdis, "Covalently cross-linked colloidosomes," Macromolecules, vol. 43, no. 24, pp. 10466-10474, 2010. View Article
[19] H. Wang, X. Zhu, L. Tsarkova, A. Pich and M. Möller, "All-Silica Colloidosomes with a Particle-Bilayer Shell," ACS Nano, vol. 5, no. 5, pp. 3937-3942, 2011. View Article
[20] A. Popadyuk, N. Solomko, A. Voronov, O. Budishevska, S. Varvarenko, V. Samaryk and S. Voronov, "Peroxidized Pickering Emulsions and Colloidosomes Thereof," Reports of the National Academy of Science of Ukraine, 2012.
[21] A. San Miguel, J. Scrimgeour, J. E. Curtis and H. Sven, "Smart colloidosomes with dissolution trigger," Soft Matter, no. 14, pp. 3136-3166, 2010. View Article
[22] K. Zhang, W. Wu, K. Guo, J. F. Chen and P. Y. Zhang, "Magnetic polymer enhanced hybrid capsules prepared from a novel Pickering emulsion polymerization and their application in controlled drug release," Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 349, no. 1-3, pp. 110-116, 2009. View Article
[23] S. Shilpi, A. Jain, Y. Gupta and S. K. Jain, "Colloidosomes: an emerging vesicular system in drug delivery," Critical Reviews in Therapeutic Drug Carrier Systems, vol. 24, no. 4, pp. 361-391, 2007. View Article
[24] F. Porta and A. Kros, "Colloidosomes as Single Implantable Beads for the In Vivo Delivery of Hydrophobic Drugs," Particle & Particle Systems Characterization, vol. 30, no. 7, pp. 606-613, 2013. View Article
[25] Y. Zhao, Y. Pan, N. Nitin and R. V. Tikekar, "Enhanced stability of curcumin in colloidosomes stabilized by silica aggregates," LWT - Food Science and Technology, vol. 58, no. 2, pp. 667-671, 2014. View Article
[26] S. P. Vyas and R. K. Khar, Targeted and Controlled Drug Delivery: Novel Carrier Systems, New Delhi: CBS Publisher, 2002. View Book
[27] V. Samaryk, I. Tarnavchyk, A. Voronov, S. Varvarenko, N. Nosova, A. Kohut and S. Voronov, "A New Acrylamide-Based Peroxide Monomer: Synthesis and Copolymerization with Octyl Methacrylate," Macromolecules, vol. 42, no. 17, pp. 6495-6500, 2009. View Article

