Volume 6 - Year 2018 - Pages 5-10
DOI: 10.11159/ijtan.2018.002
Phase Transition in TlS, TlSe and TlInS2 Crystals Caused by Nanoscale Defects
Rauf Sardarly 1, Arzu Sardarli 2, Famin Salmanov 1, Nurana Aliyeva 1, Samira Gahramanova 1, Mahammed Yusifov 1
1 Institute of Radiation Problems, National Academy of Sciences of Azerbaijan
Baku, Vahabzade 9, AZ1143, Azerbaijan Republic
sardarli@yahoo.com; famin-salmanov@rambler.ru; nur.elizade@rambler.ru;
samira_qahramanova@mail.ru; y-mehemmed1985@list.ru
2 First Nations University of Canada
1301 Central Ave, Prince Albert, SK, S6V 4W1, Canada
asardarli@fnuniv.ca
Abstract - In this paper, we report the results of our studies on temperature and electric field dependences of conductivity in TlS, TlSe and TlInS2 crystals. At a certain temperature (typical for all three crystals) a switching effect has been observed for the conductivities of all crystals. The switching effect is explained by the phase transition to superionic conductivity. It is suggested that in TlS and TlSe crystals the ion conductivity is caused by the diffusion of Tl+ ions over vacancies in the thallium sub-lattice between (Tl3+S2-2) and (Tl3+Se2-2) chains. In TlInS2 crystals, this effect is due the diffusion of Tl+ ions towards vacancies in the Tl sub-lattice. In all crystals the S-type switching effect is revealed. It is suggested that the switching effect is related to the transition of crystals to the superionic state, which is accompanied by diffusion of Tl+ ions.
Keywords: Superionic conductivity, Crystal, Phase transition, Nanoscale, Low-dimensional, Switching effect.
© Copyright 2018 Authors - This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2017-10-22
Date Accepted: 2018-03-23
Date Published: 2018-05-01
Introduction
Materials with quasi low-dimensional crystalline structure, have been objects of intensive study due their promising physical properties caused by nanoscale effects. These materials have been largely used in the fabrication of miniature accumulators, ionistors, gas sensors, solid-state fuel elements, and other devices. Superionic conductivity is one of the unique phenomenon observed in quasi low-dimensional crystals. Superionic conductivity exists under the following conditions: the network of channels in the crystal’s structure must be strong enough to withstand moving ions; the energy of ion disordering over lattice positions and the energy lost during motion should be small; the number of potentially mobile ions per unit cell should exceed the number of immobile ions [1]. These conditions are satisfied for only a few crystals, the structure of which excludes long-range ordering in the spatial arrangement of one or several types of atoms but retains long-range order for other particles. These compounds are considered crystals with intrinsic structural disordering [2]-[5]. Crystals with structural disordering, which have mainly ion conductivity, can be in two qualitatively different (dielectric and superionic) states. At temperatures below critical, they behave like conventional ionic crystals (dielectric phase), while at temperatures above critical they pass into a peculiar superionic state (electrolytic phase). This effect was studied in TlInSe2 and TlInTe2 crystals [6]. It was observed a jump in the dependences σ(T) for both crystals at some critical temperatures (in the range of 333 K – 391 K, varying with respect to the crystals and crystal axis), where σ is conductivity, and T is the absolute temperature. For both crystals, in the range of the sharp jump the dependence ln(σT) vs. 103/T was linear. The observed jumps in the conductivity was explained by the sharp change in the number of ions in the states characterized by high ion mobility, i.e., by the phase transition to the superionic state [3], [6], [7].
TlS, TlSe and TlInS2 belong to this class of materials, in which the features of low dimensional systems manifest themselves under certain conditions [1]. These crystals are attracting attention because of the features of their crystal structure, more specifically, the pronounced chain (TlS and TlSe) and layered (TlInS2) structures [8]. Weak links between chains (in TlS and TlSe) and layers (in TlInS2) result in these structures being inclined to nanoscale defects. For example, even in single crystals of this class of compounds, the density of uncontrolled defects can reach 1020 cm-3. In this case, crystals exhibit hopping conductivity similar to that observed in amorphous or highly disordered crystals, which is well described within the Mott approximation.
TlS and TlSe crystals belong to
compounds of the A3B6 group, which crystallize into
tetragonal system with the space group D184h. A
characteristic feature of these crystals is that their structure is formed by ![]() and
and ![]() chains, elongated along the tetragonal
axis c of the crystal (Fig. 1.2, [1]). The tetragonal axis is an optical axis.
Monovalent atoms are in the octahedral
environment of S and Se atoms in the TlS and TlSe crystals, respectively. Based
on the crystal-chemical considerations, it can be suggested that this crystal
structure is most favourable for mobile
chains, elongated along the tetragonal
axis c of the crystal (Fig. 1.2, [1]). The tetragonal axis is an optical axis.
Monovalent atoms are in the octahedral
environment of S and Se atoms in the TlS and TlSe crystals, respectively. Based
on the crystal-chemical considerations, it can be suggested that this crystal
structure is most favourable for mobile ![]() ions. In this case, the favourable
factor is the presence of extensive cavities, which are linked by shared faces
(conductivity windows), as well as the fundamental possibility of deficit of
monovalent thallium ions, as a result of which the ionic conductivity may
significantly rise.
ions. In this case, the favourable
factor is the presence of extensive cavities, which are linked by shared faces
(conductivity windows), as well as the fundamental possibility of deficit of
monovalent thallium ions, as a result of which the ionic conductivity may
significantly rise.
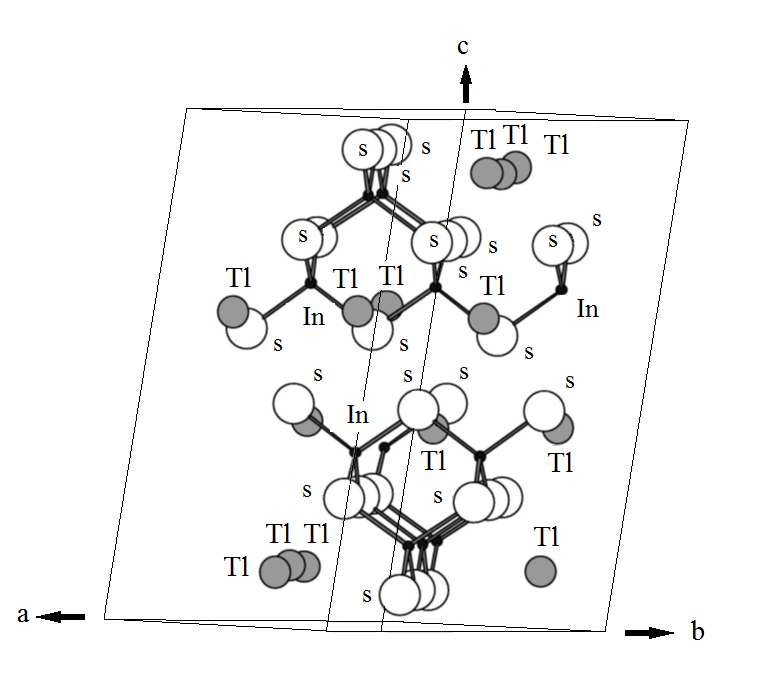
TlInS2 is a p-type
semiconductor with a layers structure made of In4S10
(which in turn consist of four tetrahedrons InS4) groups that form
layers extending perpendicular to the c-axis of the material (Fig.1). These negatively
charged layers are bonded together by ![]() ions. The resulting monoclinic
lattice is characterized by group symmetry. Significant attention has been paid
to such crystallographic systems, that behave as if they have less than three
spatial dimensions [1]-[5], [9], [10]. Such materials are often called
quasi-two-dimensional (2D) layers.
ions. The resulting monoclinic
lattice is characterized by group symmetry. Significant attention has been paid
to such crystallographic systems, that behave as if they have less than three
spatial dimensions [1]-[5], [9], [10]. Such materials are often called
quasi-two-dimensional (2D) layers.
In this paper, we report the results of experimental study of conductivity and switching effects in TlS, TlSe and TlInS2 crystals in a wide temperature range.
2. Material and Method
2.1. Material
We used Tl and S components for synthesizing TlS; Tl and Se components for synthesizing TlSe; Tl, In and S components for synthesizing TlInS2 compounds. All components had the purity no less than 99.99%. The corresponding components in the required ratio of masses for each compound were placed in evacuated quartz cells.
2.2. Method
The single crystals were grown by the modified Bridgman method [11]. The tetragonal axis c of the freshly cleaved rectangular crystal samples prepared for study was oriented in the cleavage plane. The conductivity was measured by the four-contact method in two directions: parallel and perpendicular to the tetragonal axis c of the crystal. The experimental samples were prepared in the form of rectangular plates, 0.4–0.6 mm thick. Contacts with the samples were formed by a silver conducting paste on the plate surface. The permittivity and conductivity were measured by Е7-25, digital immittance meters at frequencies of 20–106 Hz in a temperature range of 100–450 K. The measuring field amplitude did not exceed 1 V cm–1. The temperature dependences of conductivity σ(T), in TlS, TlSe, and TlInS2 are shown in Figs. 2, 3 and 4 respectively. The measurements were performed in an electric field oriented perpendicular to the tetragonal crystal axis σ⏊ (T) and parallel to it σII(T).
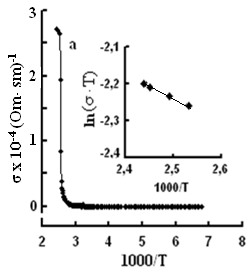
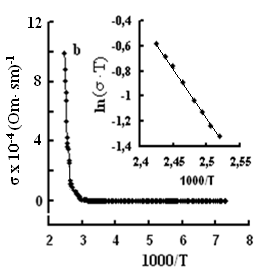
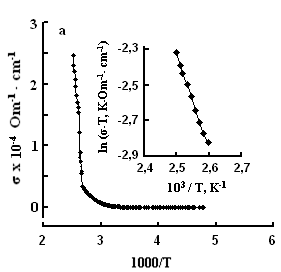
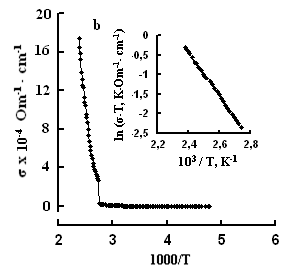
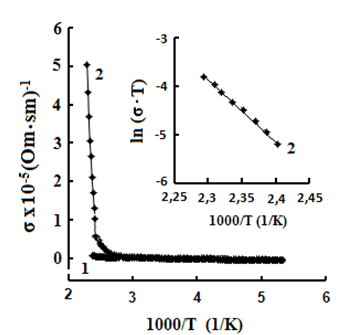
One can see jumps in the dependences σ(T) of all three crystals: for TlS a jump in σII(T)) is observed at a temperature of TIIcr = 385 K and a jump on σ(T) at T⏊cr = 387 K; for TlSe the corresponding values are TIIcr=367 K and T⏊cr =380 K; for TlInS2 the corresponding value is T⏊cr =390 K.
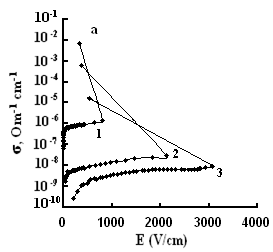
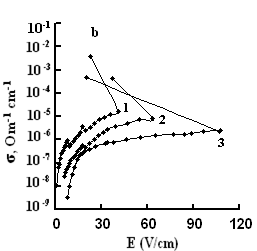
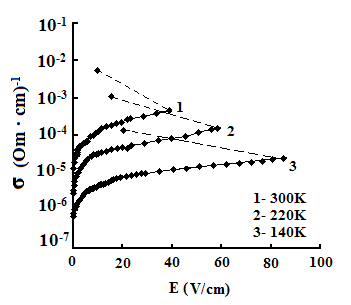
The dependences of conductivities of TlS, TlSe, and TlInS2 crystals on the electric field strength E at different temperatures are shown in Fig. 5. The measurements were performed along the tetragonal crystal axis c and perpendicular to it.
2.1. Discussions
As follows from the insets of Figs. 2, 3 and 4, for all crystals, the experimental points of the temperature dependence ln(σ·T) in the range of the sharp jump of conductivity are described well by a straight line, which is given by the following equation:
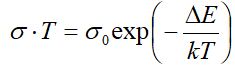
where s is the conductivity of sample, s0 is the constant within the experimental interval of temperature, ΔE is the conductivity activation energy, T is the temperature in Kelvins and k is the Boltzmann constant. It is known that this temperature dependence of conductivity indicates the dominant character of ionic conductivity above the critical temperature [2–5]. The values of critical temperatures and activation energies for TlS, TlSe, and TlInS2 crystals are shown in Table 1.
Table 1. Critical temperatures and activation energies for TlS, TlSe and TlInS2.
| Crystals | TIIcr, K | T^cr, K | DEII, eV | DE^, eV |
| TlS | 385 | 388 | 0.07 | 0.05 |
| TlSe | 367 | 380 | 0.1 | 0.07 |
| TlInS2 | N/A | 390 | 0.08 |
The observed jumps in conductivity
at critical temperatures can be explained by the sharp change in the number of
ions in the states characterized by high ion mobility, i.e., by the phase
transition to the superionic state. The linear character of the dependence ln(σ·T)
on 1/T above the critical temperature indicates dominance of ionic
conductivity, which was clearly reasoned in [2]-[5]. This reasoning can also be
strengthen by considering the structural aspect, i.e. by the existence of
channels of conductivity along the chains and layers. It is suggested that in
TlS and TlSe crystals the ion conductivity is caused by the diffusion of Tl+
ions over vacancies in the thallium sub-lattice between ![]() and
and ![]() chains. In TlInS2
crystals, this effect is due the diffusion of the
chains. In TlInS2
crystals, this effect is due the diffusion of the ![]() ions towards vacancies in the Tl
sub-lattice between In4S10
tetrahedral complexes (fig. 1). Therefore, the observed jump in the
conductivity, the linear character of the dependence ln(σ·T) vs. 1/T,
along with the structural factor, allow us to confirm the existence of superionic
conductivity in these crystals.
ions towards vacancies in the Tl
sub-lattice between In4S10
tetrahedral complexes (fig. 1). Therefore, the observed jump in the
conductivity, the linear character of the dependence ln(σ·T) vs. 1/T,
along with the structural factor, allow us to confirm the existence of superionic
conductivity in these crystals.
In all crystals, the S-type switching effect is revealed. It is suggested that the switching effect is related to the transition of crystals to the superionic state, which is accompanied by diffusion of Tl+ ions. This conduction mechanism is typical of superionic conductors [6], [9], [12], [13]. The transition to the high–conductivity state in superionic conductors occurs generally as a result of the first order phase transition and is explained by the stepwise disordering of one of the crystal sub-lattices (generally cationic) with the other sub-lattice either remaining invariable or transforming but retaining the crystal hardness. This effect was found experimentally in α-AgSbS2, where application of an external electric field caused gradual increase in conductivity with a subsequent sharp increase when the field reached the critical value [10].
As one can see from the field dependences of the conductivity (Fig. 5), in relatively weak fields, conductivity σ is almost independent of the applied field E because of the dominance of the electronic component in σ in this range of field strengths. A further increase in E led to a linear increase in σ, which is explained by the increase in the ionic component of conductivity as a result of gradual ordering of the cationic Tl sub-lattice in the electric field; in this range, the ionic conductivity begins to dominate over the electronic component and, when reaching the critical temperature, the conductivity sharply rises.
The values of the critical transition field and the jump of conductivity during the phase transition increase with a decrease in temperature (Fig. 5). Voltage oscillations were observed in the range of negative differential resistance in both compounds; these were also revealed in TlInS2.
In our opinion, the discovered effect of S type switching in the TlS, TlSe and TlInS2 crystals, as well as the voltage oscillations in the range of negative differential resistance, are related to the transition of these crystals to the superionic state, which is accompanied by melting of the Tl sub-lattice.
The measured dependences of the TlS
and TlSe conductivities on the electric field strength E indicate that,
at a certain value of the critical field (at a temperature T = 300 K, Ecr
=800, and 41 and 40 V/cm for TlS, TlSe and TlInS2, respectively),
the ![]() ion sub-lattice may undergo step
wise disordering, which is accompanied by a step wise change in conductivity.
We find it possible that the applied field causes disordering (melting) of the
cationic sub-lattice, which leads to a stepwise increase in the occupancy of
interstitial sites throughout the crystal volume.
ion sub-lattice may undergo step
wise disordering, which is accompanied by a step wise change in conductivity.
We find it possible that the applied field causes disordering (melting) of the
cationic sub-lattice, which leads to a stepwise increase in the occupancy of
interstitial sites throughout the crystal volume.
3. Conclusion
Our studies demonstrate that the temperature dependences of the conductivity of TlS, TlSe, and TlInS2 crystals indicate the dominant character of ionic conductivity above the critical temperatures. We explain this effect through the sharp change in the number of ions in the states characterized by high ion mobility, i.e., by the phase transition to the superionic state. It is remarkable that for all three crystals this phase transition occurs approximately at 380 K. We have evaluated the values of the activation energy for ionic conductivity in TlS, TlSe, and TlInS2 crystals.
The electric field dependences of the conductivity reveal the effect of S type switching in these crystals. This effect can be explained by the stepwise increase in the occupancy of interstitial sites throughout the crystal volume under the applied electric field.
In our opinion, due this effect, TlS, TlSe, and TlInS2 crystals could be considered promising materials for fabricating nano-size switches.
Acknowledgment
The authors thank Dr. Adil Abdullayev for very helpful discussions of the experimental results.
References
[1] A. M. Panich and R. M. Sardarly, Physical Properties of the Low Dimensional A3B6 and A3B3 Compounds, New York: Nova Science, 2010.
[2] L. S. Parfenyeva, A. I. Shelykh, A. I. Smirnov, A. V. Prokofyev, V. Assmus, Kh. Misiorek, Ya. Mukha, A. Ezhovskii, and I. G. Vasilyeva, “Heat Transport over Nonmagnetic Lithium Chains in LiCuVO4, a New One-Dimensional Superionic Conductor,” Physics of the Solid State, vol. 45, 2003, pp. 2093-2098. View Article
[3] Yu. Ya. Gurevich and Yu. I. Kharkats, “Features of The Thermodynamics of Superionic Conductors,” Soviet Physics Uspekhi, vol. 25, 1982, pp. 257-276.
[4] Yu. Ya. Gurevich and A. K. Ivanov, “Electronic Currents in Solid Electrolytes,” Elektrokhimiya, vol. 16, 1980, pp. 3-22. (in Russian)
[5] L. S. Parfenyev, A. I. Shelykh, I. A. Smirnov, A. V. Prokofyev, and V. Assmus, “Electrical Conductivity and Permittivity of the One-Dimensional Superionic Conductor LiCuVO4,” Physics of the Solid State, vol. 46, 2004, pp. 1027-1029. View Article
[6] R. M. Sardarly, O. A. Samedov, A. P. Abdullaev, F. T. Salmanov, O. Z. Alekperov, E. K. Huseynov, and N. A. Aliyeva, “Superionic Conductivity and Switching Effect with Memory in TlInSe2 and TlInTe2 Crystals,” Semiconductors, vol. 45, no. 11, 2011, pp. 1387-1390. View Article
[7] J. Maier and W. Miinch, “Thermal Destiny of an Ionic Crystal,” Zeitschrift für Anorganische und Allgemeine Chemie, vol. 626, 2000, pp. 264-269.
[8] W. Henkel, H. Hochheimer, C. Carlone, A. Werner, S. Ves, H. v. Schnering, “High-pressure Raman Study of The Ternary Chalcogenides T1GaS2, T1GaSe2, T1InS2, and TllnSe2,” Physical Review B, 26, 1982, pp. 3211-3221. View Article
[9] V. I. Valyukenas, A. S. Orlyukas, A. P. Sakalas, and V. A. Mikolaitis, “Influence of the External Electrical field to the Eletroconductivity of - AgSbS2,” Fiz. Tverdogo Tela, vol. 21, 1979, pp. 2449-2450. (in Russian)
[10] A. Orliukas, V. Valiukenas, V. Kybartas, A. Kezionis, “Peculiarities of Phase Transitions in α and β-AgSbS2 Single Crystals,” Ferroelectrics, vol. 38, 1981, pp. 897-900. View Article
[11] D. Chen, C. Lin, A. Maljuk, F. Zhou, Growth and Characterization of Bulk Superconductor Material. Springer Series in Materials Science, 243, 2016.
[12] R. Sardarly, O. Samedov, A. Abdullayev, F. Salmanov, A. Urbanovic, F. Garet, and J.-L. Coutaz, “Superionic Conductivity in One-Dimensional Nanofibrous TlGaTe2 Crystals,” Japanese Journal of Applied Physics, vol. 50, 2011, pp. 05FC09-1 - 05FC09-2.
[13] R. M. Sardarli, O. A. Samedov, A. P. Abdullayev, E. K. Huseynov, E. M. Qocayev, and F. T. Salmanov, “Superionic Conductivity in TlGaTe2 Crystals,” Semiconductors, vol. 45, no. 8, 2011, pp. 975-979. View Article

